Repetitive elements in aging and neurodegeneration
- PMID: 36935218
- PMCID: PMC10121923
- DOI: 10.1016/j.tig.2023.02.008
Repetitive elements in aging and neurodegeneration
Abstract
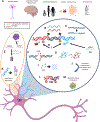
Repetitive elements (REs), such as transposable elements (TEs) and satellites, comprise much of the genome. Here, we review how TEs and (peri)centromeric satellite DNA may contribute to aging and neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS). Alterations in RE expression, retrotransposition, and chromatin microenvironment may shorten lifespan, elicit neurodegeneration, and impair memory and movement. REs may cause these phenotypes via DNA damage, protein sequestration, insertional mutagenesis, and inflammation. We discuss several TE families, including gypsy, HERV-K, and HERV-W, and how TEs interact with various factors, including transactive response (TAR) DNA-binding protein 43 kDa (TDP-43) and the siRNA and piwi-interacting (pi)RNA systems. Studies of TEs in neurodegeneration have focused on Drosophila and, thus, further examination in mammals is needed. We suggest that therapeutic silencing of REs could help mitigate neurodegenerative disorders.
Keywords: aging; neurodegeneration; satellites; transposable elements.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests K.E.C. has nothing to declare. J.S. is a consultant for Dewpoint Therapeutics, ADRx, and Neumora, and is a shareholder and advisor at Confluence Therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

