Undiagnosed diseases: Needs and opportunities in 20 countries participating in the Undiagnosed Diseases Network International
- PMID: 36935719
- PMCID: PMC10017550
- DOI: 10.3389/fpubh.2023.1079601
Undiagnosed diseases: Needs and opportunities in 20 countries participating in the Undiagnosed Diseases Network International
Abstract
Introduction: Rare diseases (RD) are a health priority worldwide, overall affecting hundreds of millions of people globally. Early and accurate diagnosis is essential to support clinical care but remains challenging in many countries, especially the low- and medium-income ones. Hence, undiagnosed RD (URD) account for a significant portion of the overall RD burden.
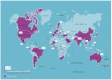
Methods: In October 2020, the Developing Nations Working Group of the Undiagnosed Diseases Network International (DNWG-UDNI) launched a survey among its members, belonging to 20 countries across all continents, to map unmet needs and opportunities for patients with URD. The survey was based on questions with open answers and included eight different domains. Conflicting interpretations were resolved in contact with the partners involved.
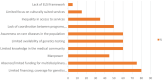
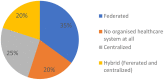
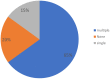
Results: All members responded to the survey. The results indicated that the scientific and medical centers make substantial efforts to respond to the unmet needs of patients. In most countries, there is a high awareness of RD issues. Scarcity of resources was highlighted as a major problem, leading to reduced availability of diagnostic expertise and research. Serious equity in accessibility to services were highlighted both within and between participating countries. Regulatory problems, including securing informed consent, difficulties in sending DNA to foreign laboratories, protection of intellectual property, and conflicts of interest on the part of service providers, remain issues of concern. Finally, most respondents stressed the need to strengthen international cooperation in terms of data sharing, clinical research, and diagnostic expertise for URD patients in low and medium income countries.
Discussion: The survey highlighted that many countries experienced a discrepancy between the growing expertise and scientific value, the level of awareness and commitment on the part of relevant parties, and funding bodies. Country-tailored public health actions, including general syllabus of medical schools and of the education of other health professionals, are needed to reduce such gaps.
Keywords: Undiagnosed Diseases; data sharing; developing nations; rare diseases; survey.
Copyright © 2023 Taruscio, Salvatore, Lumaka, Carta, Cellai, Ferrari, Sciascia, Groft, Alanay, Azam, Baynam, Cederroth, Cutiongco-de la Paz, Dissanayake, Giugliani, Gonzaga-Jauregui, Hettiarachchi, Kvlividze, Landoure, Makay, Melegh, Ozbek, Puri, Romero, Scaria, Jamuar, Shotelersuk, Roccatello, Gahl, Wiafe, Bodamer and Posada.
Conflict of interest statement
SW was employed by Rare Disease Ghana Initiative. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Regulation (EC) no 141/2000 of the European Parliament and of the Council of 16 December 1999 on Orphan Medicinal Products . Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32000R0141... (accessed October 18, 2022).
-
- Public Law 107-280 November 2002 . An act to amend the Public Health Service Act to establish an Office of Rare Diseases at the National Institutes of Health, and for other purposes. Available online at: https://www.govinfo.gov/app/details/PLAW-107publ280#:~:text=An%20act%20t...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

