Overview of the structure and function of the dopamine transporter and its protein interactions
- PMID: 36935752
- PMCID: PMC10020207
- DOI: 10.3389/fphys.2023.1150355
Overview of the structure and function of the dopamine transporter and its protein interactions
Abstract
The dopamine transporter (DAT) plays an integral role in dopamine neurotransmission through the clearance of dopamine from the extracellular space. Dysregulation of DAT is central to the pathophysiology of numerous neuropsychiatric disorders and as such is an attractive therapeutic target. DAT belongs to the solute carrier family 6 (SLC6) class of Na+/Cl- dependent transporters that move various cargo into neurons against their concentration gradient. This review focuses on DAT (SCL6A3 protein) while extending the narrative to the closely related transporters for serotonin and norepinephrine where needed for comparison or functional relevance. Cloning and site-directed mutagenesis experiments provided early structural knowledge of DAT but our contemporary understanding was achieved through a combination of crystallization of the related bacterial transporter LeuT, homology modeling, and subsequently the crystallization of drosophila DAT. These seminal findings enabled a better understanding of the conformational states involved in the transport of substrate, subsequently aiding state-specific drug design. Post-translational modifications to DAT such as phosphorylation, palmitoylation, ubiquitination also influence the plasma membrane localization and kinetics. Substrates and drugs can interact with multiple sites within DAT including the primary S1 and S2 sites involved in dopamine binding and novel allosteric sites. Major research has centered around the question what determines the substrate and inhibitor selectivity of DAT in comparison to serotonin and norepinephrine transporters. DAT has been implicated in many neurological disorders and may play a role in the pathology of HIV and Parkinson's disease via direct physical interaction with HIV-1 Tat and α-synuclein proteins respectively.
Keywords: allosteric modulators; dopamine transporter; inward-open; oligomerization; outward-open; post translational modifications; tat; α-synuclein.
Copyright © 2023 Nepal, Das, Reith and Kortagere.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

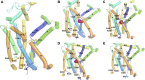
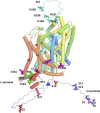
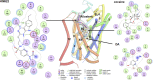
References
-
- Afonso-Oramas D., Cruz-Muros I., Alvarez de la Rosa D., Abreu P., Giraldez T., Castro-Hernandez J., et al. (2009). Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson's disease. Neurobiol. Dis. 36, 494–508. 10.1016/j.nbd.2009.09.002 - DOI - PubMed
-
- Aksenova M. V., Silvers J. M., Aksenov M. Y., Nath A., Ray P. D., Mactutus C. F., et al. (2006). HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: Changes in dopamine transporter binding and immunoreactivity. Neurosci. Lett. 395, 235–239. 10.1016/j.neulet.2005.10.095 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

