Western diet-induced shifts in the maternal microbiome are associated with altered microRNA expression in baboon placenta and fetal liver
- PMID: 36935840
- PMCID: PMC10012127
- DOI: 10.3389/fcdhc.2022.945768
Western diet-induced shifts in the maternal microbiome are associated with altered microRNA expression in baboon placenta and fetal liver
Abstract
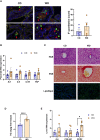
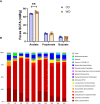
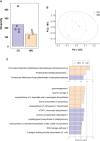
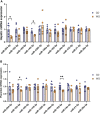
Maternal consumption of a high-fat, Western-style diet (WD) disrupts the maternal/infant microbiome and contributes to developmental programming of the immune system and nonalcoholic fatty liver disease (NAFLD) in the offspring. Epigenetic changes, including non-coding miRNAs in the fetus and/or placenta may also underlie this risk. We previously showed that obese nonhuman primates fed a WD during pregnancy results in the loss of beneficial maternal gut microbes and dysregulation of cellular metabolism and mitochondrial dysfunction in the fetal liver, leading to a perturbed postnatal immune response with accelerated NAFLD in juvenile offspring. Here, we investigated associations between WD-induced maternal metabolic and microbiome changes, in the absence of obesity, and miRNA and gene expression changes in the placenta and fetal liver. After ~8-11 months of WD feeding, dams were similar in body weight but exhibited mild, systemic inflammation (elevated CRP and neutrophil count) and dyslipidemia (increased triglycerides and cholesterol) compared with dams fed a control diet. The maternal gut microbiome was mainly comprised of Lactobacillales and Clostridiales, with significantly decreased alpha diversity (P = 0.0163) in WD-fed dams but no community-wide differences (P = 0.26). At 0.9 gestation, mRNA expression of IL6 and TNF in maternal WD (mWD) exposed placentas trended higher, while increased triglycerides, expression of pro-inflammatory CCR2, and histological evidence for fibrosis were found in mWD-exposed fetal livers. In the mWD-exposed fetus, hepatic expression levels of miR-204-5p and miR-145-3p were significantly downregulated, whereas in mWD-exposed placentas, miR-182-5p and miR-183-5p were significantly decreased. Notably, miR-1285-3p expression in the liver and miR-183-5p in the placenta were significantly associated with inflammation and lipid synthesis pathway genes, respectively. Blautia and Ruminococcus were significantly associated with miR-122-5p in liver, while Coriobacteriaceae and Prevotellaceae were strongly associated with miR-1285-3p in the placenta; both miRNAs are implicated in pathways mediating postnatal growth and obesity. Our findings demonstrate that mWD shifts the maternal microbiome, lipid metabolism, and inflammation prior to obesity and are associated with epigenetic changes in the placenta and fetal liver. These changes may underlie inflammation, oxidative stress, and fibrosis patterns that drive NAFLD and metabolic disease risk in the next generation.
Keywords: NAFLD; epigenetic programming; fetal programming; inflammation; nonhuman primate.
Conflict of interest statement
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

