Comparative complete scheme and booster effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infections with SARS-CoV-2 Omicron (BA.1) and Delta (B.1.617.2) variants: A case-case study based on electronic health records
- PMID: 36935845
- PMCID: PMC10014519
- DOI: 10.1111/irv.13121
Comparative complete scheme and booster effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infections with SARS-CoV-2 Omicron (BA.1) and Delta (B.1.617.2) variants: A case-case study based on electronic health records
Abstract
Background: Information on vaccine effectiveness in a context of novel variants of concern (VOC) emergence is of key importance to inform public health policies. This study aimed to estimate a measure of comparative vaccine effectiveness between Omicron (BA.1) and Delta (B.1.617.2 and sub-lineages) VOC according to vaccination exposure (primary or booster).
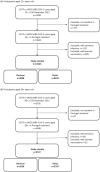
Methods: We developed a case-case study using data on RT-PCR SARS-CoV-2-positive cases notified in Portugal during Weeks 49-51, 2021. To obtain measure of comparative vaccine effectiveness, we compared the odds of vaccination in Omicron cases versus Delta using logistic regression adjusted for age group, sex, region, week of diagnosis, and laboratory of origin.
Results: Higher odds of vaccination were observed in cases infected by Omicron VOC compared with Delta VOC cases for both complete primary vaccination (odds ratio [OR] = 2.1; 95% confidence interval [CI]: 1.8 to 2.4) and booster dose (OR = 5.2; 95% CI: 3.1 to 8.8), equivalent to reduction of vaccine effectiveness from 44.7% and 92.8%, observed against infection with Delta, to -6.0% (95% CI: 29.2% to 12.7%) and 62.7% (95% CI: 35.7% to 77.9%), observed against infection with Omicron, for complete primary vaccination and booster dose, respectively.
Conclusion: Consistent reduction in vaccine-induced protection against infection with Omicron was observed. Complete primary vaccination may not be protective against SARS-CoV-2 infection in regions where Omicron variant is dominant.
Keywords: COVID‐19; Delta variant; Omicron variant; SARS‐CoV‐2; case–case design; vaccine effectiveness.
© 2023 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Peralta‐Santos reports to participate as a speaker in scientific meetings sponsored by Pfizer. Other authors report no potential conflicts of interest.
Figures
References
-
- Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS‐CoV‐2 Omicron variant in southern Africa. Nature. 2022;603(7902):679‐686. Available from: https://pubmed.ncbi.nlm.nih.gov/35042229/ - PMC - PubMed
-
- World Health Organization . Classification of Omicron (B.1.1.529): SARS‐CoV‐2 Variant of Concern. Vol. 337, WHO. 2021. [cited 2022 Jan 31]. Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1....
-
- Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS‐CoV‐2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv. 2021. Dec 2 [cited 2022 Jan 31];2021.11.11.21266068. Available from: https://www.medrxiv.org/content/10.1101/2021.11.11.21266068v2 - DOI - PMC - PubMed
-
- Karim SSA, Karim QA. Omicron SARS‐CoV‐2 variant: a new chapter in the COVID‐19 pandemic. Lancet (London, England). 2021;398(10317):2126‐2128. Available from: https://pubmed.ncbi.nlm.nih.gov/34871545/ - PMC - PubMed
-
- European Centre for Disease Prevention and Control (ECDC) . Methods for the detection and characterisation of SARS‐CoV‐2 variants—first update 20 Dec 2021. 2021.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous