High-yield vesicle-packaged recombinant protein production from E. coli
- PMID: 36936078
- PMCID: PMC10014274
- DOI: 10.1016/j.crmeth.2023.100396
High-yield vesicle-packaged recombinant protein production from E. coli
Abstract
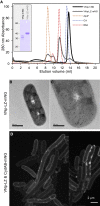
We describe an innovative system that exports diverse recombinant proteins in membrane-bound vesicles from E. coli. These recombinant vesicles compartmentalize proteins within a micro-environment that enables production of otherwise challenging insoluble, toxic, or disulfide-bond containing proteins from bacteria. The release of vesicle-packaged proteins supports isolation from the culture and allows long-term storage of active protein. This technology results in high yields of vesicle-packaged, functional proteins for efficient downstream processing for a wide range of applications from discovery science to applied biotechnology and medicine.
Keywords: disulfide bond; extracellular vesicles; recombinant protein.
© 2023 The Authors.
Conflict of interest statement
C.L. is an employee at Fujifilm-Diosynth Biotechnologies UK, Ltd. The VNp technology described here is associated with patent application #GB2118435.3.
Figures



References
-
- Kim J.H., Lee J., Park J., Gho Y.S. Gram-negative and gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015;40:97–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

