A multicenter comparative study of the performance of four rapid immunochromatographic tests for the detection of anti- Trypanosoma cruzi antibodies in Brazil
- PMID: 36936214
- PMCID: PMC10017777
- DOI: 10.3389/fmed.2023.1031455
A multicenter comparative study of the performance of four rapid immunochromatographic tests for the detection of anti- Trypanosoma cruzi antibodies in Brazil
Abstract
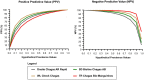
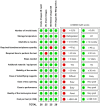
Diagnosis of Trypanosoma cruzi (T. cruzi) infection in the chronic phase of Chagas disease (CD) is performed by serologic testing. Conventional tests are currently used with very good results but require time, laboratory infrastructure, and expertise. Rapid diagnostic tests (RDTs) are an alternative as the results are immediate and do not require specialized knowledge, making them suitable for epidemiologic studies and promising as a screening tool. Nevertheless, few studies conducted comparative evaluations of RDTs to validate the results and assess their performance. In this study, we analyzed four trades of rapid tests (OnSite Chagas Ab Combo Rapid Test-United States, SD Bioline Chagas AB-United States, WL Check Chagas-Argentina, and TR Chagas Bio-Manguinhos-Brazil) using a panel of 190 samples, including sera from 111 infected individuals, most of whom had low T. cruzi antibody levels. An additional 59 samples from uninfected individuals and 20 sera from individuals with other diseases, mainly visceral leishmaniasis, were included. All tests were performed by three independent laboratories in a blinded manner. Results showed differences in sensitivity from 92.8 to 100%, specificity from 78.5 to 92.4%, and accuracy from 90.5 to 95.3% among the four assays. The results presented here show that all four RDTs have high overall diagnostic ability. However, WL Check Chagas and TR Chagas Bio-Manguinhos were considered most suitable for use in screening studies due to their high sensitivity combined with good performance. Although these two RDTs have high sensitivity, a positive result should be confirmed with other tests to confirm or rule out reactivity/positivity, especially considering possible cross-reactivity with individuals with leishmaniasis or toxoplasmosis.
Keywords: Chagas disease; performance; rapid diagnostic tests; screening; serology.
Copyright © 2023 Iturra, Leony, Medeiros, Souza Filho, Siriano, Tavares, Luquetti, Belo, Sousa and Santos.
Conflict of interest statement
AS and FS are employees of FIOCRUZ and one of the RDTs was produced by a subsidiary of FIOCRUZ (Bio-Manguinhos), but they are not involved in the production of this kit (TR Chagas Bio-Manguinhos). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- WHO . Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. (2015) 90:33–43. - PubMed
LinkOut - more resources
Full Text Sources

