Importance of high-quality evidence regarding the use of Bacopa monnieri in dementia
- PMID: 36936504
- PMCID: PMC10014812
- DOI: 10.3389/fnagi.2023.1134775
Importance of high-quality evidence regarding the use of Bacopa monnieri in dementia
Abstract
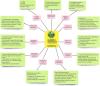
Background: Bacopa monnieri (BM), a commonly used herb, has shown neuroprotective effects in animal and in vitro studies; but human studies on patients with Alzheimer's Disease (AD) have been inconclusive. Further high-quality trials are required to conclusively state the utility of BM in AD and other neurodegenerative dementias.
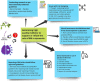
Methods: In the present study, we did a narrative review of the current challenges in designing clinical trials of BM in dementia and their evidence-based recommendations.
Results: Many facets of the BM trials need improvement, especially effect size and sample size estimation. Current assessment and outcomes measures need a more holistic approach and newer scales for diagnosing and monitoring prodromal AD. The stringent guidelines in CONSORT and STROBE are often considered difficult to implement for clinical trials in ayurvedic medications like BM. However, adherence to these guidelines will undoubtedly improve the quality of evidence and go a long way in assessing whether BM is efficacious in treating AD/prodromal AD patients and other neurodegenerative dementias.
Conclusion: Future studies on BM should implement more randomized controlled trials (RCTs) with an appropriate sample size of accurately diagnosed AD/prodromal AD patients, administering a recommended dosage of BM and for a pre-specified time calculated to achieve adequate power for the study. Researchers should also develop and validate more sensitive cognitive scales, especially for prodromal AD. BM should be evaluated in accordance with the same rigorous standards as conventional drugs to generate the best quality evidence.
Keywords: Alzheimer’s disease; Bacopa monnieri; Brahmi; dementia; evidence based medicine; high-quality evidence; mild cognitive impairment.
Copyright © 2023 Agarwal, Mishra, Gupta, Srivastava, Basheer, Sharma and Vishnu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Use of Bacopa monnieri in the Treatment of Dementia Due to Alzheimer Disease: Systematic Review of Randomized Controlled Trials.Interact J Med Res. 2022 Aug 1;11(2):e38542. doi: 10.2196/38542. Interact J Med Res. 2022. PMID: 35612544 Free PMC article. Review.
-
Efficacy of Bacopa Monnieri (Brahmi) and Donepezil in Alzheimer's Disease and Mild Cognitive Impairment: A Randomized Double-Blind Parallel Phase 2b Study.Ann Indian Acad Neurol. 2020 Nov-Dec;23(6):767-773. doi: 10.4103/aian.AIAN_610_19. Epub 2020 Dec 18. Ann Indian Acad Neurol. 2020. PMID: 33688125 Free PMC article.
-
Souvenaid for Alzheimer's disease.Cochrane Database Syst Rev. 2020 Dec 15;12(12):CD011679. doi: 10.1002/14651858.CD011679.pub2. Cochrane Database Syst Rev. 2020. PMID: 33320335 Free PMC article.
-
Protective effects of Bacopa monnieri on ischemia-induced cognitive deficits in mice: the possible contribution of bacopaside I and underlying mechanism.J Ethnopharmacol. 2015 Apr 22;164:37-45. doi: 10.1016/j.jep.2015.01.041. Epub 2015 Feb 7. J Ethnopharmacol. 2015. PMID: 25660331
-
Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract.J Ethnopharmacol. 2014;151(1):528-35. doi: 10.1016/j.jep.2013.11.008. Epub 2013 Nov 16. J Ethnopharmacol. 2014. PMID: 24252493 Review.
Cited by
-
Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis.Antioxidants (Basel). 2024 Mar 25;13(4):393. doi: 10.3390/antiox13040393. Antioxidants (Basel). 2024. PMID: 38671841 Free PMC article. Review.
-
The antioxidant potential of bacoside and its derivatives in Alzheimer's disease: The molecular mechanistic paths and therapeutic prospects.Toxicol Rep. 2025 Feb 4;14:101945. doi: 10.1016/j.toxrep.2025.101945. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 39996037 Free PMC article. Review.
References
-
- Alzheimer’s Disease Neuroimaging Initiative [ADNI] (2017). Alzheimer’s Disease Neuroimaging Initiative. Available online at: https://adni.loni.usc.edu/ (accessed July 3, 2022).
Publication types
LinkOut - more resources
Full Text Sources

