Effect of age, sex, and morbidity count on trial attrition: meta-analysis of individual participant level data from phase 3/4 industry funded clinical trials
- PMID: 36936559
- PMCID: PMC9978693
- DOI: 10.1136/bmjmed-2022-000217
Effect of age, sex, and morbidity count on trial attrition: meta-analysis of individual participant level data from phase 3/4 industry funded clinical trials
Abstract
Objectives: To estimate the association between individual participant characteristics and attrition from randomised controlled trials.
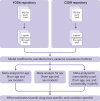
Design: Meta-analysis of individual participant level data (IPD).
Data sources: Clinical trial repositories (Clinical Study Data Request and Yale University Open Data Access).
Eligibility criteria for selecting studies: Eligible phase 3 or 4 trials identified according to prespecified criteria (PROSPERO CRD42018048202).
Main outcome measures: Association between comorbidity count (identified using medical history or concomitant drug treatment data) and trial attrition (failure for any reason to complete the final trial visit), estimated in logistic regression models and adjusted for age and sex. Estimates were meta-analysed in bayesian linear models, with partial pooling across index conditions and drug classes.
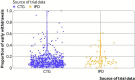
Results: In 92 trials across 20 index conditions and 17 drug classes, the mean comorbidity count ranged from 0.3 to 2.7. Neither age nor sex was clearly associated with attrition (odds ratio 1.04, 95% credible interval 0.98 to 1.11; and 0.99, 0.93 to 1.05, respectively). However, comorbidity count was associated with trial attrition (odds ratio per additional comorbidity 1.11, 95% credible interval 1.07 to 1.14). No evidence of non-linearity (assessed via a second order polynomial) was seen in the association between comorbidity count and trial attrition, with minimal variation across drug classes and index conditions. At a trial level, an increase in participant comorbidity count has a minor impact on attrition: for a notional trial with high level of attrition in individuals without comorbidity, doubling the mean comorbidity count from 1 to 2 translates to an increase in trial attrition from 29% to 31%.
Conclusions: Increased comorbidity count, irrespective of age and sex, is associated with a modest increased odds of participant attrition. The benefit of increased generalisability of including participants with multimorbidity seems likely to outweigh the disadvantages of increased attrition.
Keywords: clinical trial; internal medicine; medicine; research design.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Chief Scientist Office (Scotland), Wellcome Trust, and UK Medical Research Council for the submitted work; outside the submitted work, JSL has received personal honorariums from Bristol Myers Squibb, Pfizer, and Astra Zeneca.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
