Plexin-B1 Mutation Drives Metastasis in Prostate Cancer Mouse Models
- PMID: 36936664
- PMCID: PMC10019359
- DOI: 10.1158/2767-9764.CRC-22-0480
Plexin-B1 Mutation Drives Metastasis in Prostate Cancer Mouse Models
Abstract
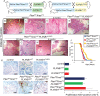
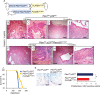
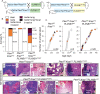
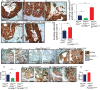
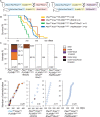
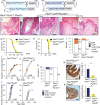
Metastatic prostate cancer is essentially incurable and is a leading cause of cancer-related morbidity and mortality in men, yet the underlying molecular mechanisms are poorly understood. Plexins are transmembrane receptors for semaphorins with divergent roles in many forms of cancer. We show here that prostate epithelial cell-specific expression of a mutant form of Plexin-B1 (P1597L) which was identified in metastatic deposits in patients with prostate cancer, significantly increases metastasis, in particular metastasis to distant sites, in two transgenic mouse models of prostate cancer (PbCre+Ptenfl /flKrasG12V and PbCre+Ptenfl /flp53fl/ fl ). In contrast, prostate epithelial cell-specific expression of wild-type (WT) Plexin-B1 in PbCre+Ptenfl /flKrasG12V mice significantly decreases metastasis, showing that a single clinically relevant Pro1597Leu amino-acid change converts Plexin-B1 from a metastasis-suppressor to a metastasis-promoter. Furthermore, PLXNB1P1597L significantly increased invasion of tumor cells into the prostate stroma, while PLXNB1WT reduced invasion, suggesting that Plexin-B1 has a role in the initial stages of metastasis. Deletion of RhoA/C or PDZRhoGEF in Ptenfl /flKrasG12VPLXNB1P1597L mice suppressed metastasis, implicating the Rho/ROCK pathway in this phenotypic switch. Germline deletion of Plexin-B1, to model anti-Plexin-B1 therapy, significantly decreased invasion and metastasis in both models. Our results demonstrate that Plexin-B1 plays a complex yet significant role in metastasis in mouse models of prostate cancer and is a potential therapeutic target to block the lethal spread of the disease.
Significance: Few therapeutic targets have been identified specifically for preventing locally invasive/oligometastatic prostate cancer from becoming more widely disseminated. Our findings suggest Plexin-B1 signaling, particularly from the clinically relevant P1597L mutant, is such a target.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol 2000;10:377–83. - PubMed
-
- Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol 2005;6:789–800. - PubMed
-
- Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem 2008;283:1893–901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

