Pentafluorosulfanyl-functionalised BODIPY push-pull dyes for p-type dye-sensitized solar cells
- PMID: 36936698
- PMCID: PMC10013097
- DOI: 10.1039/d2se00977c
Pentafluorosulfanyl-functionalised BODIPY push-pull dyes for p-type dye-sensitized solar cells
Abstract
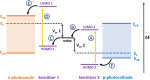
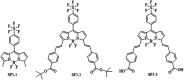
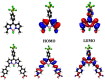
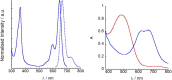
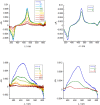
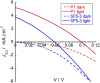
We report a push-pull BODIPY-based dye functionalised with an electronegative SF5 group at the meso position for applications in photocathodes in tandem dye-sensitized solar cells (DSSCs). The push-pull character enhances charge-transfer from the mesoporous NiO cathode surface towards the redox mediator. A Knoevenagel condensation reaction was used to introduce the carboxylic acid to anchor the dye to the oxide surface, via a styryl linker which increases the conjugation in the molecule and shifts the absorption to the red. The room-temperature synthesis and high yields, make the dye promising for manufacture on a large scale. The dye was applied in p-DSSCs giving a power conversion efficiency (0.066%), a short circuit photocurrent (J SC) of 3.84 mA cm-2, open circuit voltage (V OC) of 58 mV and fill factor of 30%.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Push-Pull Phenoxazine-Based Sensitizers for p-Type DSSCs: Effect of Acceptor Units on Photovoltaic Performance.ChemSusChem. 2022 Aug 19;15(16):e202200520. doi: 10.1002/cssc.202200520. Epub 2022 Jul 1. ChemSusChem. 2022. PMID: 35691936
-
Enhanced Photocurrent Density by Spin-Coated NiO Photocathodes for N-Annulated Perylene-Based p-Type Dye-Sensitized Solar Cells.ACS Appl Mater Interfaces. 2016 Aug 3;8(30):19393-401. doi: 10.1021/acsami.6b04007. Epub 2016 Jul 25. ACS Appl Mater Interfaces. 2016. PMID: 27416960
-
Application of the tris(acetylacetonato)iron(III)/(II) redox couple in p-type dye-sensitized solar cells.Angew Chem Int Ed Engl. 2015 Mar 16;54(12):3758-62. doi: 10.1002/anie.201409877. Epub 2015 Jan 28. Angew Chem Int Ed Engl. 2015. PMID: 25631105
-
New bithiazole-based sensitizers for efficient and stable dye-sensitized solar cells.Chemistry. 2012 Jun 18;18(25):7903-15. doi: 10.1002/chem.201103702. Epub 2012 May 9. Chemistry. 2012. PMID: 22573564
-
Redox Shuttle-Based Electrolytes for Dye-Sensitized Solar Cells: Comprehensive Guidance, Recent Progress, and Future Perspective.ACS Omega. 2023 Feb 6;8(7):6139-6163. doi: 10.1021/acsomega.2c06843. eCollection 2023 Feb 21. ACS Omega. 2023. PMID: 36844550 Free PMC article. Review.
Cited by
-
Self-Assembled Monolayers of Push-Pull Chromophores as Active Layers and Their Applications.Molecules. 2024 Jan 23;29(3):559. doi: 10.3390/molecules29030559. Molecules. 2024. PMID: 38338304 Free PMC article. Review.
References
-
- O'Regan B. Grätzel M. Nature. 1991;353:737–740. doi: 10.1038/353737a0. - DOI
-
- He J. Lindström H. Hagfeldt A. Lindquist S. J. Phys. Chem. B. 1999;103:8940–8943. doi: 10.1021/jp991681r. - DOI
-
- He J. Lindström H. Hagfeldt A. Lindquist S.-E. Sol. Energy Mater. Sol. Cells. 2000;62:265–273. doi: 10.1016/S0927-0248(99)00168-3. - DOI
LinkOut - more resources
Full Text Sources
