An image-based high-content screening for compounds targeting Toxoplasma gondii repurposed inhibitors effective against the malaria parasite Plasmodium falciparum
- PMID: 36936758
- PMCID: PMC10020723
- DOI: 10.3389/fcimb.2023.1102551
An image-based high-content screening for compounds targeting Toxoplasma gondii repurposed inhibitors effective against the malaria parasite Plasmodium falciparum
Abstract
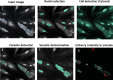
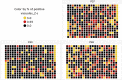
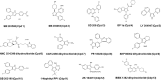
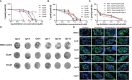
Apicomplexa phylum includes numerous obligate intracellular protozoan parasites that are life threatening for humans and animals. In this context, Plasmodium falciparum and Toxoplasma gondii are of particular interest, as they are responsible for malaria and toxoplasmosis, respectively, for which efficient vaccines are presently lacking and therapies need to be improved. Apicomplexan parasites have a highly polarized morphology, with their apical end containing specific secretory organelles named rhoptries and micronemes, which depend on the unique receptor and transporter sortilin TgSORT for their biogenesis. In the present study, we took advantage of the subcellular polarity of the parasite to engineer a clonal transgenic Toxoplasma line that expresses simultaneously the green fluorescent protein TgSORT-GFP in the post-Golgi-endosome-like compartment and the red fluorescent protein rhoptry ROP1-mCherry near the apical end. We utilized this fluorescent transgenic T. gondii to develop a miniaturized image-based phenotype assay coupled to an automated image analysis. By applying this methodology to 1,120 compounds, we identified 12 that are capable of disrupting the T. gondii morphology and inhibiting intracellular replication. Analysis of the selected compounds confirmed that all 12 are kinase inhibitors and intramembrane pumps, with some exhibiting potent activity against Plasmodium falciparum. Our findings highlight the advantage of comparative and targeted phenotypic analysis involving two related parasite species as a means of identifying molecules with a conserved mode of action.
Keywords: ROP1-mCherry; TgSORT-GFP; Toxoplasma gondii; high-content screening; image-based analysis; malaria parasite; repurposing drugs.
Copyright © 2023 Honfozo, Houngue, Vandeputte, Dechavanne, Nouatin, Atindehou, Fanou, Massougbodji, Dechavanne, Brodin and Tomavo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Butcher B. A., Fox B. A., Rommereim L. M., Kim S. G., Maurer K. J., Yarovinsky F., et al. . (2011). Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PloS Pathog. 7, e1002236. doi: 10.1371/journal.ppat.1002236 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

