Mycobacterium tuberculosis-macrophage interaction: Molecular updates
- PMID: 36936766
- PMCID: PMC10020944
- DOI: 10.3389/fcimb.2023.1062963
Mycobacterium tuberculosis-macrophage interaction: Molecular updates
Abstract
Mycobacterium tuberculosis (Mtb), the causative agent of Tuberculosis (TB), remains a pathogen of great interest on a global scale. This airborne pathogen affects the lungs, where it interacts with macrophages. Acidic pH, oxidative and nitrosative stressors, and food restrictions make the macrophage's internal milieu unfriendly to foreign bodies. Mtb subverts the host immune system and causes infection due to its genetic arsenal and secreted effector proteins. In vivo and in vitro research have examined Mtb-host macrophage interaction. This interaction is a crucial stage in Mtb infection because lung macrophages are the first immune cells Mtb encounters in the host. This review summarizes Mtb effectors that interact with macrophages. It also examines how macrophages control and eliminate Mtb and how Mtb manipulates macrophage defense mechanisms for its own survival. Understanding these mechanisms is crucial for TB prevention, diagnosis, and treatment.
Keywords: Mycobacterium tuberculosis; host macrophage; immune control; immune evasion; intracellular pathogen; molecular interaction; tuberculosis control.
Copyright © 2023 Bo, Moure, Yang, Pan, Li, Wang, Ke and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

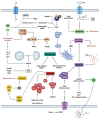
References
-
- Ali M. K., Zhen G., Nzungize L., Stojkoska A., Duan X., Li C., et al. (2020). Mycobacterium tuberculosis PE31 (Rv3477) attenuates host cell apoptosis and promotes recombinant m. smegmatis intracellular survival via up-regulating GTPase guanylate binding protein-1. Front. Cell Infect. Microbiol. 10, 40. doi: 10.3389/fcimb.2020.00040 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

