Non-muscle myosin II drives critical steps of nematocyst morphogenesis
- PMID: 36936784
- PMCID: PMC10014300
- DOI: 10.1016/j.isci.2023.106291
Non-muscle myosin II drives critical steps of nematocyst morphogenesis
Abstract
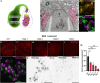
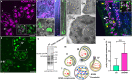
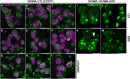
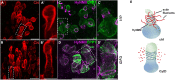
Nematocysts are generated by secretion of proteins into a post-Golgi compartment. They consist of a capsule that elongates into a long tube, which is coiled inside the capsule matrix and expelled during its nano-second discharge deployed for prey capture. The driving force for discharge is an extreme osmotic pressure of 150 bar. The complex processes of tube elongation and invagination under these biomechanical constraints have so far been elusive. Here, we show that a non-muscle myosin II homolog (HyNMII) is essential for nematocyst formation in Hydra. In early nematocysts, HyNMII assembles to a collar around the neck of the protruding tube. HyNMII then facilitates tube outgrowth by compressing it along the longitudinal axis as evidenced by inhibitor treatment and genetic knockdown. In addition, live imaging of a NOWA::NOWA-GFP transgenic line, which re-defined NOWA as a tube component facilitating invagination, allowed us to analyze the impact of HyNMII on tube maturation.
Keywords: Cell biology; Developmental biology.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources

