Fibroblast exosomal TFAP2C induced by chitosan oligosaccharides promotes peripheral axon regeneration via the miR-132-5p/CAMKK1 axis
- PMID: 36936807
- PMCID: PMC10020534
- DOI: 10.1016/j.bioactmat.2023.03.002
Fibroblast exosomal TFAP2C induced by chitosan oligosaccharides promotes peripheral axon regeneration via the miR-132-5p/CAMKK1 axis
Abstract
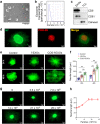
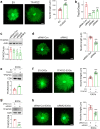
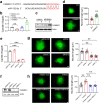
Chitosan and its degradation product, oligosaccharides, have been shown to facilitate peripheral nerve regeneration. However, the underlying mechanisms are not well understood. In this study, we analyzed the protein expression profiles in sciatic nerves after injury using proteomics. A group of proteins related to exosome packaging and transport is up-regulated by chitosan oligosaccharides (COS), implying that exosomes are involved in COS-induced peripheral nerve regeneration. In fact, exosomes derived from fibroblasts (f-EXOs) treated with COS significantly promoted axon extension and regeneration. Exosomal protein identification and functional studies, revealed that TFAP2C is a key factor in neurite outgrowth induced by COS-f-EXOs. Furthermore, we showed that TFAP2C targets the pri-miRNA-132 gene and represses miR-132-5p expression in dorsal root ganglion neurons. Camkk1 is a downstream substrate of miR-132-5p that positively affects axon extension. In rats, miR-132-5p antagomir stimulates CAMKK1 expression and improves axon regeneration and functional recovery in sciatic nerves after injury. Our data reveal the mechanism for COS in axon regeneration, that is COS induce fibroblasts to produce TFAP2C-enriched EXOs, which are then transferred into axons to promote axon regeneration via miR-132-5p/CAMKK1. Moreover, these results show a new facet of fibroblasts in axon regeneration in peripheral nerves.
Keywords: TFAP2C; axon regeneration; chitosan oligosaccharides; fibroblast exosomes; peripheral nerves.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflict of interests.
Figures







References
-
- Millesi H. Techniques for nerve grafting. Hand Clin. 2000;16:73–91. viii. - PubMed
LinkOut - more resources
Full Text Sources

