Antiviral activity of amide-appended α-hydroxytropolones against herpes simplex virus-1 and -2
- PMID: 36936842
- PMCID: PMC10016935
- DOI: 10.1039/d2ra06749h
Antiviral activity of amide-appended α-hydroxytropolones against herpes simplex virus-1 and -2
Abstract
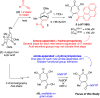
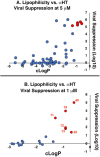
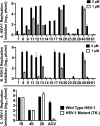
α-Hydroxytropolones (αHTs) have potent antiviral activity against herpes simplex virus-1 and -2 (HSV-1 and HSV-2) in cell culture, including against acyclovir-resistant mutants, and as a result have the potential to be developed as antiviral drugs targeting these viruses. We recently described a convenient final-step amidation strategy to their synthesis, and this was used to generate 57 amide-substituted αHTs that were tested against hepatitis B virus. The following manuscript describes the evaluation of this library against HSV-1, as well as a subset against HSV-2. The structure-function analysis obtained from these studies demonstrates the importance of lipophilicity and rigidity to αHT-based anti-HSV potency, consistent with our prior work on smaller libraries. We used this information to synthesize and test a targeted library of 4 additional amide-appended αHTs. The most potent of this new series had a 50% effective concentration (EC50) for viral inhibition of 72 nM, on par with the most potent αHT antivirals we have found to date. Given the ease of synthesis of amide-appended αHTs, this new class of antiviral compounds and the chemistry to make them should be highly valuable in future anti-HSV drug development.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
LAM and RPM are inventors on patents describing the HSV antiviral activity of αHTs.
Figures
References
-
- Whitley R. J., Herpes Simplex Viruses, in Fields Virology, ed. D. Knipe and P. Howley, Lippincott Williams & Wilkins, Philadelphia, PA, US, 4th edn, 2001, vol. 2, pp. 2461–2509
LinkOut - more resources
Full Text Sources

