CAR-iNKT cells targeting clonal TCRVβ chains as a precise strategy to treat T cell lymphoma
- PMID: 36936927
- PMCID: PMC10019783
- DOI: 10.3389/fimmu.2023.1118681
CAR-iNKT cells targeting clonal TCRVβ chains as a precise strategy to treat T cell lymphoma
Abstract
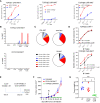
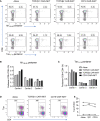
Introduction: Most T cell receptor (TCR)Vβ chain-expressing T cell lymphomas (TCL) including those caused by Human T cell leukaemia virus type-1 (HTLV-1) have poor prognosis. We hypothesised that chimeric antigen receptor (CAR)-mediated targeting of the clonal, lymphoma-associated TCRβ chains would comprise an effective cell therapy for TCL that would minimally impact the physiological TCR repertoire.
Methods: As proof of concept, we generated CAR constructs to target four TCRVβ subunits. Efficacy of the CAR constructs was tested using conventional T cells as effectors (CAR-T). Since invariant NKT (iNKT) cell do not incite acute graft-versus-host disease and are suitable for 'off-the-shelf' immunotherapy, we generated anti-TCRVβ CAR-iNKT cells.
Results: We show that anti-TCRVβ CAR-T cells selectively kill their cognate tumour targets while leaving >90% of the physiological TCR repertoire intact. CAR-iNKT cells inhibited the growth of TCL in vivo, and were also selectively active against malignant cells from Adult T cell leukaemia/lymphoma patients without activating expression of HTLV-1.
Discussion: Thus we provide proof-of-concept for effective and selective anti-TCRVβ CAR-T and -iNKT cell-based therapy of TCL with the latter providing the option for 'off-the-shelf' immunotherapy.
Keywords: ATL; T cell lymphoma; T cell receptor; adult T cell leukaemia/lymphoma; chimeric antigen receptor (CAR) T-cells; human T cell leukaemia virus type-1; human T cell lymphotropic virus type-1 (HTLV-1); iNKT.
Copyright © 2023 Rowan, Ponnusamy, Ren, Taylor, Cook and Karadimitris.
Conflict of interest statement
AK has received consultancy fees from Arovella Therapeutics. LC has received honoraria from Abbvie and Roche. AK and AR have filed for patent based on the work presented here. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Tembhare P, Yuan CM, Xi L, Morris JC, Liewehr D, Venzon D, et al. Flow cytometric immunophenotypic assessment of T-cell clonality by vβ repertoire analysis: Detection of T-cell clonality at diagnosis and monitoring of minimal residual disease following therapy. Am J Clin Pathol (2011) 135:890–900. doi: 10.1309/AJCPV2D1DDSGJDBW - DOI - PMC - PubMed
-
- Rowan AG, Witkover A, Melamed A, Tanaka Y, Cook LBM, Fields P, et al. T Cell receptor vβ staining identifies the malignant clone in adult T cell leukemia and reveals killing of leukemia cells by autologous CD8+ T cells. PloS Pathog (2016) 12:e1006030. doi: 10.1371/journal.ppat.1006030 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

