Megakaryocytes respond during sepsis and display innate immune cell behaviors
- PMID: 36936945
- PMCID: PMC10019826
- DOI: 10.3389/fimmu.2023.1083339
Megakaryocytes respond during sepsis and display innate immune cell behaviors
Abstract
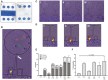
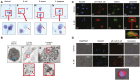
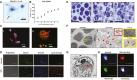
Megakaryocytes (MKs) are precursors to platelets, the second most abundant cells in the peripheral circulation. However, while platelets are known to participate in immune responses and play significant functions during infections, the role of MKs within the immune system remains largely unexplored. Histological studies of sepsis patients identified increased nucleated CD61+ cells (MKs) in the lungs, and CD61+ staining (likely platelets within microthrombi) in the kidneys, which correlated with the development of organ dysfunction. Detailed imaging cytometry of peripheral blood from patients with sepsis found significantly higher MK counts, which we predict would likely be misclassified by automated hematology analyzers as leukocytes. Utilizing in vitro techniques, we show that both stem cell derived MKs (SC MKs) and cells from the human megakaryoblastic leukemia cell line, Meg-01, undergo chemotaxis, interact with bacteria, and are capable of releasing chromatin webs in response to various pathogenic stimuli. Together, our observations suggest that MK cells display some basic innate immune cell behaviors and may actively respond and play functional roles in the pathophysiology of sepsis.
Keywords: infectious; innate; megakaryocyte; platelet; sepsis.
Copyright © 2023 Frydman, Ellett, Jorgensen, Marand, Zukerberg, Selig, Tessier, Wong, Olaleye, Vanderburg, Fox, Tompkins and Irimia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Yacoub A, Brockman A. Extramedullary hematopoiesis and splenic vein thrombosis, a unique presentation of pre-clinical essential thrombocythemia. Blood (2013) 122(21):5257. doi: 10.1182/blood.V122.21.5257.5257 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

