Skeletal myotube-derived extracellular vesicles enhance itaconate production and attenuate inflammatory responses of macrophages
- PMID: 36936950
- PMCID: PMC10018131
- DOI: 10.3389/fimmu.2023.1099799
Skeletal myotube-derived extracellular vesicles enhance itaconate production and attenuate inflammatory responses of macrophages
Abstract
Introduction: Macrophages play an important role in the innate immunity. While macrophage inflammation is necessary for biological defense, it must be appropriately controlled. Extracellular vesicles (EVs) are small vesicles released from all types of cells and play a central role in intercellular communication. Skeletal muscle has been suggested to release anti-inflammatory factors, but the effect of myotube-derived EVs on macrophages is unknown. As an anti-inflammatory mechanism of macrophages, the immune responsive gene 1 (IRG1)-itaconate pathway is essential. In this study, we show that skeletal muscle-derived EVs suppress macrophage inflammatory responses, upregulating the IRG1-itaconate pathway.
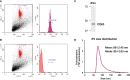
Methods: C2C12 myoblasts were differentiated into myotubes and EVs were extracted by ultracentrifugation. Skeletal myotube-derived EVs were administered to mouse bone marrow-derived macrophages, then lipopolysaccharide (LPS) stimulation was performed and inflammatory cytokine expression was measured by RT-qPCR. Metabolite abundance in macrophages after addition of EVs was measured by CE/MS, and IRG1 expression was measured by RT-PCR. Furthermore, RNA-seq analysis was performed on macrophages after EV treatment.
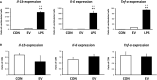
Results: EVs attenuated the expression of LPS-induced pro-inflammatory factors in macrophages. Itaconate abundance and IRG1 expression were significantly increased in the EV-treated group. RNA-seq analysis revealed activation of the PI3K-Akt and JAK-STAT pathways in macrophages after EV treatment. The most abundant miRNA in myotube EVs was miR-206-3p, followed by miR-378a-3p, miR-30d-5p, and miR-21a-5p.
Discussion: Skeletal myotube EVs are supposed to increase the production of itaconate via upregulation of IRG1 expression and exhibited an anti-inflammatory effect in macrophages. This anti-inflammatory effect was suggested to involve the PI3K-Akt and JAK-STAT pathways. The miRNA profiles within EVs implied that miR-206-3p, miR-378a-3p, miR-30d-5p, and miR-21a-5p may be responsible for the anti-inflammatory effects of the EVs. In summary, in this study we showed that myotube-derived EVs prevent macrophage inflammatory responses by activating the IRG1-itaconate pathway.
Keywords: IRG1; extracellular vesicle; itaconate; macrophage; skeletal muscle.
Copyright © 2023 Yamaguchi, Maeshige, Yan, Ma, Uemura, Matsuda, Nishimura, Hasunuma, Kondo, Fujino and Yuan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

