A systematic review and meta-analysis evaluating the impact of antibiotic use on the clinical outcomes of cancer patients treated with immune checkpoint inhibitors
- PMID: 36937417
- PMCID: PMC10019357
- DOI: 10.3389/fonc.2023.1075593
A systematic review and meta-analysis evaluating the impact of antibiotic use on the clinical outcomes of cancer patients treated with immune checkpoint inhibitors
Abstract
Background: Immune checkpoint inhibitors (ICIs) have considerably improved patient outcomes in various cancer types, but their efficacy remains poorly predictable among patients. The intestinal microbiome, whose balance and composition can be significantly altered by antibiotic use, has recently emerged as a factor that may modulate ICI efficacy. The objective of this systematic review and meta-analysis is to investigate the impact of antibiotics on the clinical outcomes of cancer patients treated with ICIs.
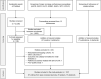
Methods: PubMed and major oncology conference proceedings were systematically searched to identify all studies reporting associations between antibiotic use and at least one of the following endpoints: Overall Survival (OS), Progression-Free Survival (PFS), Objective Response Rate (ORR) and Progressive Disease (PD) Rate. Pooled Hazard Ratios (HRs) for OS and PFS, and pooled Odds Ratios (ORs) for ORR and PD were calculated. Subgroup analyses on survival outcomes were also performed to investigate the potential differential effect of antibiotics according to cancer types and antibiotic exposure time windows.
Results: 107 articles reporting data for 123 independent cohorts were included, representing a total of 41,663 patients among whom 11,785 (28%) received antibiotics around ICI initiation. The pooled HRs for OS and PFS were respectively of 1.61 [95% Confidence Interval (CI) 1.48-1.76] and 1.45 [95% CI 1.32-1.60], confirming that antibiotic use was significantly associated with shorter survival. This negative association was observed consistently across all cancer types for OS and depending on the cancer type for PFS. The loss of survival was particularly strong when antibiotics were received shortly before or after ICI initiation. The pooled ORs for ORR and PD were respectively of 0.59 [95% CI 0.47-0.76] and 1.86 [95% CI 1.41-2.46], suggesting that antibiotic use was significantly associated with worse treatment-related outcomes.
Conclusion: As it is not ethically feasible to conduct interventional, randomized, controlled trials in which antibiotics would be administered to cancer patients treated with ICIs to demonstrate their deleterious impact versus control, prospective observational studies and interventional trials involving microbiome modifiers are crucially needed to uncover the role of microbiome and improve patient outcomes. Such studies will reduce the existing publication bias by allowing analyses on more homogeneous populations, especially in terms of treatments received, which is not possible at this stage given the current state of the field. In the meantime, antibiotic prescription should be cautiously considered in cancer patients receiving ICIs.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42019145675.
Keywords: antibiotics; cancer; immune checkpoint inhibitors; immunotherapies; meta-analysis; microbiome; microbiota; systematic review.
Copyright © 2023 Crespin, Le Bescop, de Gunzburg, Vitry, Zalcman, Cervesi and Bandinelli.
Conflict of interest statement
The authors declare that this study received funding from Da Volterra, France. The funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication. AC, FV, and JC are employees of Da Volterra. CL and P-AB are employees of Da Volterra and may have owned or received shares of Da Volterra, during the study period. JG and GZ are consultants for Da Volterra and report receiving consulting fees from Da Volterra. JG reports having owned shares of Da Volterra, during the study period. GZ reports having received grants, personal fees, and nonfinancial support from ROCHE; personal fees from MSD; personal fees and non-financial support from Bristol-Myers Squibb, Inventiva, Sanofi-Regeneron and Astra-Zeneca Pharmaceuticals; and non-financial support from Pfizer, AbbVie, and Da Volterra, outside of the submitted work.
Figures









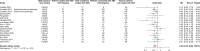
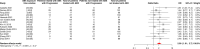
References
-
- Lo Russo G, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, et al. . Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res (2019) 25:989–99. doi: 10.1158/1078-0432.CCR-18-1390 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

