The inhibitory effect of 6-gingerol and cisplatin on ovarian cancer and antitumor activity: In silico, in vitro, and in vivo
- PMID: 36937441
- PMCID: PMC10020515
- DOI: 10.3389/fonc.2023.1098429
The inhibitory effect of 6-gingerol and cisplatin on ovarian cancer and antitumor activity: In silico, in vitro, and in vivo
Abstract
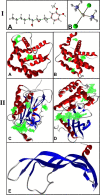
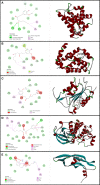
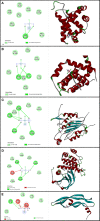
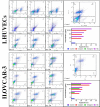
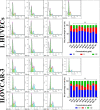
Background: Epithelial ovarian cancer is very common in women and causes hundreds of deaths per year worldwide. Chemotherapy drugs including cisplatin have adverse effects on patients' health. Complementary treatments and the use of herbal medicines can help improve the performance of medicine. 6-Gingerol is the major pharmacologically active component of ginger. In this study, we compared the effects of 6-gingerol, cisplatin, and their combination in apoptotic and angiogenetic activities in silico, in test tubes, and in in vivo assays against two ovarian cancer cell lines: OVCAR-3 and human umbilical vein endothelial cells (HUVECs).
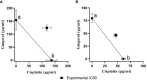
Methods: The drug-treated cell lines were evaluated for their cytotoxicity, cell cycle, and apoptotic and angiogenetic gene expression changes.
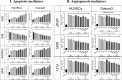
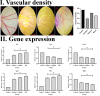
Results: The proportion of apoptosis treated by 6-gingerol coupled with cisplatin was significantly high. In the evaluation of the cell cycle, the combination therapy also showed a significant promotion of a higher extent of the S sequence. The expression of p53 level, Caspase-8, Bax, and Apaf1 genes was amplified again with combination therapy. Conversely, in both cell lines, the cumulative drug concentrations reduced the expression of VEGF, FLT1, KDR, and Bcl-2 genes. Similarly, in the control group, combination treatment significantly decreased the expression of VEGF, FLT1, KDR, and Bcl-2 genes in comparison to cisplatin alone.
Conclusions: The findings of the present study demonstrated that the cisplatin and 6-gingerol combination is more effective in inducing apoptosis and suppressing the angiogenesis of ovarian cancer cells than using each drug alone.
Keywords: angiogenesis; apoptosis; chick embryo; cisplatin; gingerol; molecular dynamics simulation; ovarian neoplasm.
Copyright © 2023 Salari, Khosravi, Pourkhandani, Molaakbari, Salarkia, Keyhani, Sharifi, Tavakkoli, Sohbati, Dabiri, Ren and Shafie’ei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Evaluation of the cytotoxicity of the Bithionol - cisplatin combination in a panel of human ovarian cancer cell lines.BMC Cancer. 2017 Jan 13;17(1):49. doi: 10.1186/s12885-016-3034-2. BMC Cancer. 2017. PMID: 28086831 Free PMC article.
-
Combination of AT-101/cisplatin overcomes chemoresistance by inducing apoptosis and modulating epigenetics in human ovarian cancer cells.Mol Biol Rep. 2013 Jun;40(6):3925-33. doi: 10.1007/s11033-012-2469-z. Epub 2012 Dec 27. Mol Biol Rep. 2013. PMID: 23269627
-
6-Gingerol Mediates its Anti Tumor Activities in Human Oral and Cervical Cancer Cell Lines through Apoptosis and Cell Cycle Arrest.Phytother Res. 2016 Apr;30(4):588-95. doi: 10.1002/ptr.5561. Epub 2016 Jan 7. Phytother Res. 2016. PMID: 26749462
-
The potent peptide antagonist to angiogenesis, C16Y, and cisplatin act synergistically in the down-regulation of the Bcl-2/Bax ratio and the induction of apoptosis in human ovarian cancer cells.Int J Oncol. 2011 Dec;39(6):1359-64. doi: 10.3892/ijo.2011.1134. Epub 2011 Jul 20. Int J Oncol. 2011. PMID: 21935568
-
Time-dependent changes in factors involved in the apoptotic process in human ovarian cancer cells as a response to cisplatin.Gynecol Oncol. 2002 Mar;84(3):404-12. doi: 10.1006/gyno.2001.6537. Gynecol Oncol. 2002. PMID: 11855878
Cited by
-
Gingerol acts as a potent radiosensitizer in head and neck squamous cell carcinoma.Discov Oncol. 2024 Oct 13;15(1):553. doi: 10.1007/s12672-024-01425-y. Discov Oncol. 2024. PMID: 39397185 Free PMC article.
-
6-Gingerol Induced Apoptosis and Cell Cycle Arrest in Glioma Cells via MnSOD and ERK Phosphorylation Modulation.Biomol Ther (Seoul). 2025 Jan 1;33(1):129-142. doi: 10.4062/biomolther.2024.084. Epub 2024 Dec 5. Biomol Ther (Seoul). 2025. PMID: 39632791 Free PMC article.
-
Blockage of Autophagy for Cancer Therapy: A Comprehensive Review.Int J Mol Sci. 2024 Jul 7;25(13):7459. doi: 10.3390/ijms25137459. Int J Mol Sci. 2024. PMID: 39000565 Free PMC article. Review.
-
6-Gingerol modulates miRNAs and PODXL gene expression via methyltransferase enzymes in NB4 cells: an in silico and in vitro study.Sci Rep. 2024 Aug 7;14(1):18356. doi: 10.1038/s41598-024-68069-4. Sci Rep. 2024. PMID: 39112503 Free PMC article.
-
Discovery of drug transporter inhibitors tied to long noncoding RNA in resistant cancer cells; a computational model -in silico- study.Front Immunol. 2025 Apr 25;16:1511029. doi: 10.3389/fimmu.2025.1511029. eCollection 2025. Front Immunol. 2025. PMID: 40352931 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

