Transauricular vagus nerve stimulation for patients with disorders of consciousness: A randomized controlled clinical trial
- PMID: 36937511
- PMCID: PMC10017768
- DOI: 10.3389/fneur.2023.1133893
Transauricular vagus nerve stimulation for patients with disorders of consciousness: A randomized controlled clinical trial
Abstract
Introduction: Disorders of consciousness (DoCs) are a frequent complication of brain injury disease, and effective treatments are currently lacking. Transauricular vagus nerve stimulation (tVNS) has been proposed as a promising therapeutic method for neurological disorders such as epilepsy and depression. In our previous study, we demonstrated that vagus nerve stimulation promoted recovery in rats with DoCs caused by traumatic brain injury. However, the clinical effect of vagus nerve stimulation on consciousness disorders is unclear. We aimed to investigate the therapeutic efficacy and safety of tVNS in patients with DoCs.
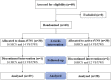
Methods: We conducted a randomized, double-blinded, sham-controlled trial. Patients (N = 60) with DoCs, including minimally conscious state (MCS) and vegetative state/unresponsive wakefulness syndrome, were enrolled and randomized to groups receiving either active or sham tVNS. A frequency of 20 Hz and pulse wave of 200 us was used in the active-tVNS protocol, which was performed in the auricular branch of the vagus nerve in the left outer ear. The sham-tVNS protocol was the same as the active-tVNS protocol although without current input. Both groups of patients also received conventional treatments. Consciousness was evaluated according to the Coma Recovery Scale-Revised before and after the 4-week intervention. We also recorded the type and number of behavioral responses. Safety was primarily assessed according to the incidence of treatment-emergent adverse events. Each patient's heart rate and blood pressure were monitored during all treatment sessions.
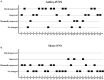
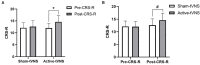
Results: Ultimately, 57 patients completed the study: 28 patients underwent active tVNS and 29 patients underwent sham tVNS. No significant differences were observed in Coma Recovery Scale-Revised scores between the active- and sham-tVNS groups before the tVNS sessions. Compared with patients in the sham-tVNS group (9.28 ± 4.38), patients with DoCs treated with active tVNS showed improved consciousness (10.93 ± 4.99), although not statistically significant. Further analysis revealed obvious differences between patients with MCS receiving active and sham tVNS, but no significant difference in patients with vegetative state/unresponsive wakefulness syndrome in both groups. All side effects were considered common medical conditions with no obvious correlation to tVNS.
Conclusion: These preliminary data provide early evidence that tVNS may be an effective and safe approach for promoting the recovery of consciousness, especially in patients with MCS.
Clinical trial registration: https://www.chictr.org.cn/edit.aspx?pid=175938&htm=4, identifier: ChiCTR2200066629.
Keywords: Coma Recovery Scale-Revised; disorders of consciousness; minimally conscious state (MCS); transauricular vagus nerve stimulation; vegetative state/unresponsive wakefulness syndrome (VS/UWS).
Copyright © 2023 Zhou, Kang, Xiong, Feng and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The efficacy and safety of transcutaneous auricular vagus nerve stimulation for patients with minimally conscious state: a sham-controlled randomized double-blind clinical trial.Front Neurosci. 2023 Dec 14;17:1323079. doi: 10.3389/fnins.2023.1323079. eCollection 2023. Front Neurosci. 2023. PMID: 38156271 Free PMC article.
-
Transcranial direct current stimulation effects in disorders of consciousness.Arch Phys Med Rehabil. 2014 Feb;95(2):283-9. doi: 10.1016/j.apmr.2013.09.002. Epub 2013 Sep 11. Arch Phys Med Rehabil. 2014. PMID: 24035769
-
Meditative-based diaphragmatic breathing vs. vagus nerve stimulation in the treatment of fibromyalgia-A randomized controlled trial: Body vs. machine.Front Neurol. 2022 Nov 3;13:1030927. doi: 10.3389/fneur.2022.1030927. eCollection 2022. Front Neurol. 2022. PMID: 36438970 Free PMC article.
-
Stimulation of vagus nerve for patients with disorders of consciousness: a systematic review.Front Neurosci. 2023 Sep 15;17:1257378. doi: 10.3389/fnins.2023.1257378. eCollection 2023. Front Neurosci. 2023. PMID: 37781261 Free PMC article. Review.
-
Behavioral effects in disorders of consciousness following transcranial direct current stimulation: A systematic review and individual patient data meta-analysis of randomized clinical trials.Front Neurol. 2022 Sep 29;13:940361. doi: 10.3389/fneur.2022.940361. eCollection 2022. Front Neurol. 2022. PMID: 36247787 Free PMC article.
Cited by
-
Vagus nerve stimulation: a physical therapy with promising potential for central nervous system disorders.Front Neurol. 2024 Dec 13;15:1516242. doi: 10.3389/fneur.2024.1516242. eCollection 2024. Front Neurol. 2024. PMID: 39734634 Free PMC article. Review.
-
The efficacy and safety of transcutaneous auricular vagus nerve stimulation for patients with minimally conscious state: a sham-controlled randomized double-blind clinical trial.Front Neurosci. 2023 Dec 14;17:1323079. doi: 10.3389/fnins.2023.1323079. eCollection 2023. Front Neurosci. 2023. PMID: 38156271 Free PMC article.
-
Transcutaneous auricular vagus nerve stimulation in the treatment of disorders of consciousness: mechanisms and applications.Front Neurosci. 2023 Oct 18;17:1286267. doi: 10.3389/fnins.2023.1286267. eCollection 2023. Front Neurosci. 2023. PMID: 37920298 Free PMC article. Review.
-
Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) for Insomnia Disorder: A Narrative Review of Effectiveness, Mechanisms and Recommendations for Clinical Practice.Nat Sci Sleep. 2025 Jun 13;17:1327-1344. doi: 10.2147/NSS.S515809. eCollection 2025. Nat Sci Sleep. 2025. PMID: 40535058 Free PMC article. Review.
-
Case report: Monitoring consciousness with fNIRS in a patient with prolonged reduced consciousness following hemorrhagic stroke undergoing adjunct taVNS therapy.Front Neurosci. 2025 Feb 4;19:1519939. doi: 10.3389/fnins.2025.1519939. eCollection 2025. Front Neurosci. 2025. PMID: 39967804 Free PMC article.
References
-
- Pistarini C, Maggioni G. Disorders of Consciousness. In:T. Platz, editor. Clinical Pathways in Stroke Rehabilitation: Evidence-Based Clinical Practice Recommendations. Cham: Springer; (2021), 57–70.
LinkOut - more resources
Full Text Sources

