Molecular Identification, Pathogenesis, and Life Cycle of Sarcocystis cruzi from Cattle (Bos taurus) in New Valley Governorate, Egypt
- PMID: 36937557
- PMCID: PMC10017224
- DOI: 10.1155/2023/7829290
Molecular Identification, Pathogenesis, and Life Cycle of Sarcocystis cruzi from Cattle (Bos taurus) in New Valley Governorate, Egypt
Abstract
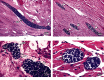
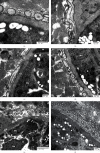
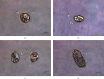
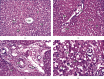
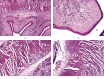
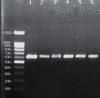
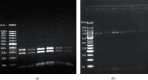
Sarcocystis cruzi was identified by molecular methods from an intermediate host, cattle (Bos taurus), in El-Kharga, New Valley Governorate, Egypt, and its life cycle and pathogenicity were studied in the final host, dogs (Canis familiaris). 600 slaughtered cattle aged 6-8 years (480/120 males/females) were included. In addition, three laboratory-bred, coccidian-free puppies aged 2-3 months were fed infected bovine muscles to locate the definitive host and analyze sporogony. 18S rRNA-specific gene primers were used for DNA amplification from esophageal muscles. These polymerase chain reaction (PCR) amplicons were subjected to restriction fragment length polymorphism (RFLP) and molecular sequence analysis. Infection was detected in 78.8% (473/600; 95% CI, 75.56-82.11%). Histopathological examination of esophageal muscles showed oval- to spherical-shaped cysts, 96.7 μm wide by 326.9 μm long; cysts in cardiac muscles were ovoid and smaller. Infected puppies began shedding sporocysts in feces 7 days post-inoculation and showed distorted organ architecture, severe cellular damage, and inflammatory lesions in liver, kidney, esophagus, and stomach. Three oocysts with different shapes and sizes were identified. Partial 18S rRNA gene sequences of isolated New Valley sarcocysts were identical to S. cruzi isolated from different areas, verifying their genetic relatedness. Our analysis suggests that S. cruzi is the most prevalent in slaughtered cattle in New Valley Governorate, Egypt.
Copyright © 2023 Mohammed B. M. El-Mahdi et al.
Conflict of interest statement
The author(s) declare(s) that they have no conflicts of interest.
Figures



Similar articles
-
Molecular differentiation of Sarcocystis buffalonis and Sarcocystis levinei in water buffaloes (Bubalus bubalis) from Sarcocystis hirsuta and Sarcocystis cruzi in cattle (Bos taurus).Parasitol Res. 2016 Jun;115(6):2459-71. doi: 10.1007/s00436-016-4998-1. Epub 2016 Mar 16. Parasitol Res. 2016. PMID: 26979729
-
Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis).Parasitol Res. 2016 Apr;115(4):1473-92. doi: 10.1007/s00436-015-4881-5. Epub 2015 Dec 17. Parasitol Res. 2016. PMID: 26677095
-
First molecular characterization of Sarcocystis spp. in cattle in Qena Governorate, Upper Egypt.J Parasit Dis. 2018 Mar;42(1):114-121. doi: 10.1007/s12639-017-0974-7. Epub 2018 Jan 12. J Parasit Dis. 2018. PMID: 29491569 Free PMC article.
-
A review of Sarcocystis of domestic animals and of other coccidia of cats and dogs.J Am Vet Med Assoc. 1976 Nov 15;169(10):1061-78. J Am Vet Med Assoc. 1976. PMID: 824260 Review.
-
Identity of Sarcocystis species of the water buffalo (Bubalus bubalis) and cattle (Bos taurus) and the suppression of Sarcocystis sinensis as a nomen nudum.Vet Parasitol. 2014 Sep 15;205(1-2):1-6. doi: 10.1016/j.vetpar.2014.06.020. Epub 2014 Jun 19. Vet Parasitol. 2014. PMID: 25034134 Review.
Cited by
-
Infection survey, molecular, pathogenicity, and morphological characteristics of Sarcocystis species naturally infected water buffaloes (Bubalus bubalis) in Egypt.BMC Vet Res. 2024 Dec 23;20(1):578. doi: 10.1186/s12917-024-04408-x. BMC Vet Res. 2024. PMID: 39716208 Free PMC article.
-
Molecular Confirmation of Raptors from Spain as Definitive Hosts of Numerous Sarcocystis Species.Animals (Basel). 2025 Feb 23;15(5):646. doi: 10.3390/ani15050646. Animals (Basel). 2025. PMID: 40075929 Free PMC article.
-
Sarcocystis species: molecular identification and seroprevalence in water buffaloes (Bubalus bubalis).BMC Vet Res. 2025 Jul 22;21(1):482. doi: 10.1186/s12917-025-04933-3. BMC Vet Res. 2025. PMID: 40696429 Free PMC article.
References
-
- Dubey J. T. A review of Sarcocystis of domestic animals and of other coccidia of cats and dogs. Journal of the American Veterinary Medical Association . 1976;169(10):1061–1078. - PubMed
-
- Dubey J. P., Speer C., Fayer R. Sarcocystosis of Animals and Man . Boca Raton: CRC Press, Inc.; 1989. p. p. 215.
LinkOut - more resources
Full Text Sources

