1-Azahomocubane
- PMID: 36937576
- PMCID: PMC10016339
- DOI: 10.1039/d3sc00001j
1-Azahomocubane
Abstract
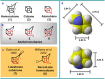
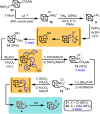
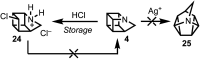
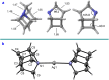
Highly strained cage hydrocarbons have long stood as fundamental molecules to explore the limits of chemical stability and reactivity, probe physical properties, and more recently as bioactive molecules and in materials discovery. Interestingly, the nitrogenous congeners have attracted much less attention. Previously absent from the literature, azahomocubanes, offer an opportunity to investigate the effects of a nitrogen atom when incorporated into a highly constrained polycyclic environment. Herein disclosed is the synthesis of 1-azahomocubane, accompanied by comprehensive structural characterization, physical property analysis and chemical reactivity. These data support the conclusion that nitrogen is remarkably well tolerated in a highly strained environment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Dunn G. L. DiPasquo V. J. Hoover J. R. E. Synthesis of pentacyclo[4.3.0.02,5.03,8.04,7]nonane and some 4-substituted derivatives. Tetrahedron Lett. 1966;7:3737–3742. doi: 10.1016/S0040-4039(01)99957-5. - DOI
-
- Eaton P. E. Cole T. W. CubaneJ. Am. Chem. Soc. 1964;86:3157–3158. doi: 10.1021/ja01069a041. - DOI
-
- Alkorta I. Elguero J. Rozas I. Balaban A. T. Theoretical studies of aza analogues of platonic hydrocarbons: Part 1. Cubane and its aza derivatives. J. Mol. Struct.: THEOCHEM. 1990;206:67–75. doi: 10.1016/0166-1280(90)85007-A. - DOI
-
- Murray J. S. Seminario J. M. Lane P. Politzer P. Anomalous energy effects associated with the presence of aza nitrogens and nitro substituents in some strained systems. J. Mol. Struct.: THEOCHEM. 1990;207:193–200. doi: 10.1016/0166-1280(90)85023-G. - DOI
LinkOut - more resources
Full Text Sources

