Synthesis of functionalised isochromans: epoxides as aldehyde surrogates in hexafluoroisopropanol
- PMID: 36937595
- PMCID: PMC10016621
- DOI: 10.1039/d2sc06692k
Synthesis of functionalised isochromans: epoxides as aldehyde surrogates in hexafluoroisopropanol
Abstract
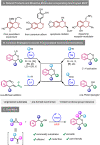
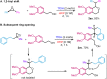
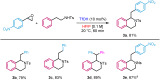
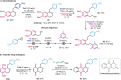
The oxa-Pictet-Spengler reaction is arguably the most straightforward and modular way to construct the privileged isochroman motif, but its scope is largely limited to benzaldehyde derivatives and to electron-rich β-phenylethanols that lack substitution along the aliphatic chain. Here we describe a variant of this reaction starting from an epoxide, rather than an aldehyde, that greatly expands the scope and rate of the reaction (<1 h, 20 °C). Besides facilitating the initial Meinwald rearrangement, the use of hexafluoroisopropanol (HFIP) as a solvent expands the electrophile scope to include partners equivalent to ketones, aliphatic aldehydes, and phenylacetyl aldehydes, and the nucleophile scope to include modestly electronically deactivated and highly substituted β-phenylethanols. The products could be easily further derivatised in the same pot by subsequent ring-opening, reductions, and intra- and intermolecular Friedel-Crafts reactions, also in HFIP. Finally, owing to the high pharmacological relevance of the isochroman motif, the synthesis of drug analogues was demonstrated.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Ferreira E. M. Nat. Chem. 2014;6:94–96. doi: 10.1038/nchem.1851. - DOI - PubMed
- Simonneau A. Oestreich M. Nat. Chem. 2015;7:816–822. doi: 10.1038/nchem.2329. - DOI - PubMed
- Zhang X. Liu Z. Yang X. Dong Y. Virelli M. Zanoni G. Anderson E. A. Bi X. Nat. Commun. 2019;10:284. doi: 10.1038/s41467-018-08253-z. - DOI - PMC - PubMed
- Zimmerman J. B. Anastas P. T. Erythropel H. C. Leitner W. Science. 2020;367:397–400. doi: 10.1126/science.aay3060. - DOI - PubMed
- Dai X.-J. Li C.-C. Li C.-J. Chem. Soc. Rev. 2021;50:10733–10742. doi: 10.1039/D1CS00418B. - DOI - PubMed
-
-
For selected reviews on HFIP, see:
- Colomer I. Chamberlain A. E. R. Haughey M. B. Donohoe T. J. Nat. Rev. Chem. 2017;1:0088. doi: 10.1038/s41570-017-0088. - DOI
- Sinha S. K. Bhattacharya T. Maiti D. React. Chem. Eng. 2019;4:244–253. doi: 10.1039/C8RE00225H. - DOI
- An X.-D. Xiao J. Chem. Rec. 2020;20:142–161. doi: 10.1002/tcr.201900020. - DOI - PubMed
- Yu C. Sanjosé-Orduna J. Patureau F. W. Pérez-Temprano M. H. Chem. Soc. Rev. 2020;49:1643–1652. doi: 10.1039/C8CS00883C. - DOI - PubMed
- Pozhydaiev V. Power M. Gandon V. Moran J. Lebœuf D. Chem. Commun. 2020;56:11548–11564. doi: 10.1039/D0CC05194B. - DOI - PubMed
- Bhattacharya T. Ghosh A. Maiti D. Chem. Sci. 2021;12:3857–3870. doi: 10.1039/D0SC06937J. - DOI - PMC - PubMed
- Motiwala H. F. Armaly A. M. Cacioppo J. G. Coombs T. C. Koehn K. R. K. Norwood IV V. M. Aubé J. Chem. Rev. 2022;122:12544–12747. doi: 10.1021/acs.chemrev.1c00749. - DOI - PubMed
-
-
- Meinwald J. Labana S. S. Chadha M. S. J. Am. Chem. Soc. 1963;85:582–585. doi: 10.1021/ja00888a022. - DOI
- Rickborn B., Acid-Catalyzed Rearrangements of Epoxides, in Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon: Oxford, 1991, vol. 3, pp. 733–775
-
- Vayer M. Zhang S. Moran J. Lebœuf D. ACS Catal. 2022;12:3309–3316. doi: 10.1021/acscatal.2c00216. - DOI
-
- Yudin A. K., Aziridines and Epoxides in Organic Synthesis, Wiley-VCH, Weinheim, 2006
LinkOut - more resources
Full Text Sources

