Discovery of a simple iron catalyst reveals the intimate steps of C-H amination to form C-N bonds
- PMID: 36937598
- PMCID: PMC10016609
- DOI: 10.1039/d2sc04170g
Discovery of a simple iron catalyst reveals the intimate steps of C-H amination to form C-N bonds
Abstract
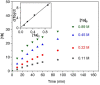
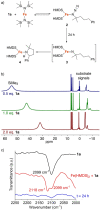
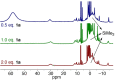
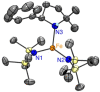
Formation of ubiquitous C-N bonds traditionally uses prefunctionalized carbon precursors. Recently, metal-catalyzed amination of unfunctionalized C-H bonds with azides has become an attractive and atom-economic strategy for C-N bond formation, though all catalysts contain sophisticated ligands. Here, we report Fe(HMDS)2 (HMDS = N(SiMe3)2 -) as an easy-to-prepare catalyst for intramolecular C-H amination. The catalyst shows unprecedented turnover frequencies (110 h-1 vs. 70 h-1 reported to date) and requires no additives. Amination is successful for benzylic and aliphatic C-H bonds (>80% yield) and occurs even at room temperature. The simplicity of the catalyst enabled for the first time comprehensive mechanistic investigations. Kinetic, stoichiometric, and computational studies unveiled the intimate steps of the C-H amination process, including the resting state of the catalyst and turnover-limiting N2 loss of the coordinated azide. The high reactivity of the iron imido intermediate is rationalized by its complex spin system revealing imidyl and nitrene character.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Catalytic C-H bond amination from high-spin iron imido complexes.J Am Chem Soc. 2011 Apr 6;133(13):4917-23. doi: 10.1021/ja110066j. Epub 2011 Mar 15. J Am Chem Soc. 2011. PMID: 21405138
-
Deciphering Iron-Catalyzed C-H Amination with Organic Azides: N2 Cleavage from a Stable Organoazide Complex.Chemistry. 2024 Jan 16;30(4):e202303410. doi: 10.1002/chem.202303410. Epub 2023 Nov 28. Chemistry. 2024. PMID: 37916523
-
Transition-metal-catalyzed C-N bond forming reactions using organic azides as the nitrogen source: a journey for the mild and versatile C-H amination.Acc Chem Res. 2015 Apr 21;48(4):1040-52. doi: 10.1021/acs.accounts.5b00020. Epub 2015 Mar 30. Acc Chem Res. 2015. PMID: 25821998
-
Selective functionalisation of saturated C-H bonds with metalloporphyrin catalysts.Chem Soc Rev. 2011 Apr;40(4):1950-75. doi: 10.1039/c0cs00142b. Epub 2011 Mar 9. Chem Soc Rev. 2011. PMID: 21387046 Review.
-
Iron-catalysed intramolecular C(sp3)-H amination of alkyl azides.Chem Commun (Camb). 2024 Nov 26;60(95):13998-14011. doi: 10.1039/d4cc04169k. Chem Commun (Camb). 2024. PMID: 39531011 Review.
Cited by
-
Application of first-row transition metal complexes bearing 1,2,3-triazolylidene ligands in catalysis and beyond.Chem Soc Rev. 2024 Jun 17;53(12):6322-6344. doi: 10.1039/d4cs00021h. Chem Soc Rev. 2024. PMID: 38726664 Free PMC article. Review.
-
From the bottle: simple iron salts for the efficient synthesis of pyrrolidines via catalytic C-H bond amination.Catal Sci Technol. 2023 Jan 25;13(4):958-962. doi: 10.1039/d2cy02065c. eCollection 2023 Feb 20. Catal Sci Technol. 2023. PMID: 36825222 Free PMC article.
-
Iron Corrole-Catalyzed Intramolecular Amination Reactions of Alkyl Azides. Spectroscopic Characterization and Reactivity of [FeV(Cor)(NAd)].Adv Sci (Weinh). 2024 Oct;11(39):e2401420. doi: 10.1002/advs.202401420. Epub 2024 Aug 20. Adv Sci (Weinh). 2024. PMID: 39162002 Free PMC article.
-
Enantioselective C-H amination catalyzed by homoleptic iron salox complexes.Chem Commun (Camb). 2025 Sep 5. doi: 10.1039/d5cc04627k. Online ahead of print. Chem Commun (Camb). 2025. PMID: 40910938 Free PMC article.
-
A Cobotic, Digitally Controlled Schlenk-line Unlocks Access to Elusive Lewis-Base Stabilised Copper Bis(Disilylamides).Angew Chem Int Ed Engl. 2025 Aug 11;64(33):e202505408. doi: 10.1002/anie.202505408. Epub 2025 Jun 17. Angew Chem Int Ed Engl. 2025. PMID: 40460195 Free PMC article.
References
-
- Ricci A., Amino Group Chemistry: From Synthesis to the Life Sciences, Wiley-VCH: Weinheim, 2008

