Endoscope reprocessing: Retrospective analysis of 90,311 samples
- PMID: 36937825
- PMCID: PMC10023244
- DOI: 10.1055/a-1991-1391
Endoscope reprocessing: Retrospective analysis of 90,311 samples
Abstract
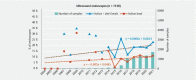
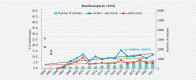
Background and study aims The contamination level of ready-to-use endoscopes published in the literature varies from 0.4 % to 49.0 %. Unfortunately, the comparison and the interpretation of these results are quite impossible, given the limited number of samples and sites included and the differences observed between sampling, culturing methods, and interpretation criteria. Methods The objective of this retrospective study was to analyze the results of 90,311 endoscope samples collected between 2004 and 2021 in 490 private or public hospitals in France. Results Through the full test period, the mean ratio of endoscopes at the action level was 12.6 % (19.5 % including alert level). Of the endoscopy units, 23.0 % had a ratio of compliant endoscopes ≤ 70.0 %. The overall microbial quality of gastroscopes, duodenoscopes, and colonoscopes is improving year by year, whereas an opposite trend is observed for ultrasound endoscopes and bronchoscopes. In 2021, following French guidelines, 13.0 % of the endoscopes should have been quarantined and 8.1 % were at the alert level, meaning that the contamination level of 21.1 % of the endoscopes exceeded what was defined as a maximum acceptable value. Conclusions This study demonstrates that additional efforts, including implementation of microbial surveillance strategies using a standardized sampling method and periodic observational audits, must be made to improve the overall microbiological quality of endoscopes and reduce the risk associated with their use.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Competing interests L Pineau is an employee of Eurofins Biotech Germande which reports having been consulted and having received financial support from medical device manufacturers to performed studies on the efficacy of medical devices.
Figures








Comment in
-
Search better and you will find more: an important lesson in endoscope contamination.Endosc Int Open. 2023 Apr 17;11(4):E366-E367. doi: 10.1055/a-2017-3933. eCollection 2023 Apr. Endosc Int Open. 2023. PMID: 37077661 Free PMC article. No abstract available.
-
Microbiological surveillance - where do we stand?Endosc Int Open. 2023 Apr 28;11(4):E443-E445. doi: 10.1055/a-2066-8222. eCollection 2023 Apr. Endosc Int Open. 2023. PMID: 37124715 Free PMC article. No abstract available.
References
-
- Beilenhoff U, Biering H, Blum R et al.ESGE-ESGENA technical specification for process validation and routine testing of endoscope reprocessing in washer-disinfectors according to EN ISO 15883, parts 1, 4, and ISO/TS 15883-5. Endoscopy. 2017;49:1262–1275. - PubMed
-
- Beilenhoff U, Biering H, Blum R et al.Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) – Update 2018. Endoscopy. 2018;50:1205–1234. - PubMed
-
- ISO 15883-4:2018. Washer-disinfectors – Part 4: Requirements and tests for washer-disinfectors employing chemical disinfection for thermolabile endoscopes. https://www.iso.org/standard/63696.html https://www.iso.org/standard/63696.html
-
- Pineau L, Desbuquois C, Marchetti B et al.Comparison of the fixative properties of five disinfectant solutions. J Hosp Infect. 2008;68:171–177. - PubMed
-
- Instruction n° DGOS/PF2/DGS/VSS1/2016/220 du 4 juillet 2016 relative au traitement des endoscopes souples thermosensibles à canaux au sein des lieux de soins. https://www.legifrance.gouv.fr/circulaire/id/41172 https://www.legifrance.gouv.fr/circulaire/id/41172
LinkOut - more resources
Full Text Sources

