A Potential Off-Target Effect of the Wnt/β-Catenin Inhibitor KYA1797K: PD-L1 Binding and Checkpoint Inhibition
- PMID: 36938364
- PMCID: PMC10015704
- DOI: 10.1159/000528499
A Potential Off-Target Effect of the Wnt/β-Catenin Inhibitor KYA1797K: PD-L1 Binding and Checkpoint Inhibition
Abstract
Introduction: The quest for small molecule inhibitors of the PD-1/PD-L1 checkpoint continues in parallel to the extensive development of monoclonal antibodies directed against this immune checkpoint. Drug screening strategies are being set up to identify novel PD-L1 inhibitors.
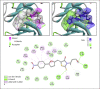
Methods: A virtual screening based on molecular docking with the PD-L1 protein dimer has been performed to identify a new binder. Binding of the identified ligand to PD-L1 has been validated experimentally using a microscale thermophoresis (MST) assay. The cellular effect of the compound was evidenced using a fluorescence resonance energy transfer (FRET) assay based on activation of tyrosine phosphatase SHP-2.
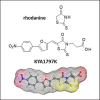
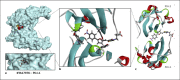
Results: We have identified the potent Wnt/β-catenin inhibitor KYA1797K as a weak PD-L1 binder. Molecular docking suggested that the compound can bind to the interface of a PD-L1 dimer, with a geometry superimposable to that of the reference PD-L1 inhibitor BMS-202. The atypical 2-thioxo-4-thiazolidinone motif of KYA1797K, derived from the natural product rhodanine, plays a major role in the interaction with PD-L1. Binding of KYA1797K to recombinant hPD-L1 was validated experimentally, using MST. The drug was found to bind modestly but effectively to hPD-L1. The FRET assay confirmed the weak capacity of KYA1797K to interfere with the activation of SHP-2 upon its interaction with human PD-1.
Discussion: Collectively, the data show that KYA1797K could function as a weak modulator of the PD-1/PD-L1 checkpoint. This effect may contribute, at least partially, to the reported capacity of the β-catenin inhibitor to downregulate PD-L1 in cancer cells. The work also underlines the interest to further consider the rhodanine moiety as a chemical motif for the design of new PD-L1 binders.
Keywords: Cancer; Drug design; Immune checkpoint; KYA1797K; PD-L1 inhibitor.
Copyright © 2023 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no relevant financial or nonfinancial interests to disclose.
Figures



References
-
- Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022;19((5)):287–305. - PubMed
-
- Wang Y, Zhang N, Wang F, Li Z. Proceedings: AACR annual meeting. Atlanta (GA): 2019. March 29–April 3, Abstract LB-018: orally active small molecule PD-L1 inhibitor demonstrating similar efficacy as Durvalumab in human knock-in MC38 mode.
LinkOut - more resources
Full Text Sources
Research Materials

