Crisscross multilayering of cell sheets
- PMID: 36938501
- PMCID: PMC10019763
- DOI: 10.1093/pnasnexus/pgad034
Crisscross multilayering of cell sheets
Abstract
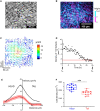
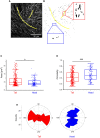
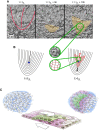
Hydrostatic skeletons such as the Hydra's consist of two stacked layers of muscle cells perpendicularly oriented. In vivo, these bilayers first assemble, and then the muscle fibers of both layers develop and organize with this crisscross orientation. In the present work, we identify an alternative mechanism of crisscross bilayering of myoblasts in vitro, which results from the prior local organization of these active cells in the initial monolayer. The myoblast sheet can be described as a contractile active nematic in which, as expected, most of the +1/2 topological defects associated with this nematic order self-propel. However, as a result of the production of extracellular matrix (ECM) by the cells, a subpopulation of these comet-like defects does not show any self-propulsion. Perpendicular bilayering occurs at these stationary defects. Cells located at the head of these defects converge toward their core where they accumulate until they start migrating on top of the tail of the first layer, while the tail cells migrate in the opposite direction under the head. Since the cells keep their initial orientations, the two stacked layers end up perpendicularly oriented. This concerted process leading to a crisscross bilayering is mediated by the secretion of ECM.
Keywords: active cell nematics; collective cell behaviors; multilayers; orientation.
© The Author(s) 2023. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures

References
LinkOut - more resources
Full Text Sources

