The molecular grammar of protein disorder guiding genome-binding locations
- PMID: 36938874
- PMCID: PMC10250222
- DOI: 10.1093/nar/gkad184
The molecular grammar of protein disorder guiding genome-binding locations
Abstract
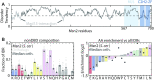
Intrinsically disordered regions (IDRs) direct transcription factors (TFs) towards selected genomic occurrences of their binding motif, as exemplified by budding yeast's Msn2. However, the sequence basis of IDR-directed TF binding selectivity remains unknown. To reveal this sequence grammar, we analyze the genomic localizations of >100 designed IDR mutants, each carrying up to 122 mutations within this 567-AA region. Our data points at multivalent interactions, carried by hydrophobic-mostly aliphatic-residues dispersed within a disordered environment and independent of linear sequence motifs, as the key determinants of Msn2 genomic localization. The implications of our results for the mechanistic basis of IDR-based TF binding preferences are discussed.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Wang C., Uversky V.N., Kurgan L.. Disordered nucleiome: abundance of intrinsic disorder in the DNA- and RNA-binding proteins in 1121 species from Eukaryota, Bacteria and Archaea. Proteomics. 2016; 16:1486–1498. - PubMed
-
- Minezaki Y., Homma K., Kinjo A.R., Nishikawa K.. Human transcription factors contain a high fraction of intrinsically disordered regions essential for transcriptional regulation. J. Mol. Biol. 2006; 359:1137–1149. - PubMed
-
- Ward J.J., Sodhi J.S., McGuffin L.J., Buxton B.F., Jones D.T.. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J. Mol. Biol. 2004; 337:635–645. - PubMed
-
- Brodsky S., Jana T., Barkai N.. Order through disorder: the role of intrinsically disordered regions in transcription factor binding specificity. Curr. Opin. Struct. Biol. 2021; 71:110–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

