Keeping It Organized: Multicompartment Constructs to Mimic Tissue Heterogeneity
- PMID: 36938891
- PMCID: PMC11469230
- DOI: 10.1002/adhm.202202110
Keeping It Organized: Multicompartment Constructs to Mimic Tissue Heterogeneity
Abstract
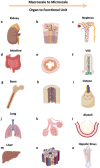
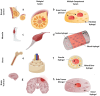
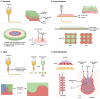
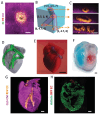
Tissue engineering aims at replicating tissues and organs to develop applications in vivo and in vitro. In vivo, by engineering artificial constructs using functional materials and cells to provide both physiological form and function. In vitro, by engineering three-dimensional (3D) models to support drug discovery and enable understanding of fundamental biology. 3D culture constructs mimic cell-cell and cell-matrix interactions and use biomaterials seeking to increase the resemblance of engineered tissues with its in vivo homologues. Native tissues, however, include complex architectures, with compartmentalized regions of different properties containing different types of cells that can be captured by multicompartment constructs. Recent advances in fabrication technologies, such as micropatterning, microfluidics or 3D bioprinting, have enabled compartmentalized structures with defined compositions and properties that are essential in creating 3D cell-laden multiphasic complex architectures. This review focuses on advances in engineered multicompartment constructs that mimic tissue heterogeneity. It includes multiphasic 3D implantable scaffolds and in vitro models, including systems that incorporate different regions emulating in vivo tissues, highlighting the emergence and relevance of 3D bioprinting in the future of biological research and medicine.
Keywords: 3D bioprinting; hydrogels; in vitro models; multicompartment models; tissue engineering.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Berthiaume F., Maguire T. J., Yarmush M. L., Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403. - PubMed
-
- Langer R., Vacanti J., inJournal of Pediatric Surgery,W. B. Saunder, Philadelphia, US: 2016, pp. 8–12.
-
- Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C. T., Biomaterials 2020, 226, 119536. - PubMed

