A phase 3 study of safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, followed by 23-valent pneumococcal polysaccharide vaccine, in children with HIV
- PMID: 36939067
- PMCID: PMC10241418
- DOI: 10.1097/QAD.0000000000003551
A phase 3 study of safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, followed by 23-valent pneumococcal polysaccharide vaccine, in children with HIV
Abstract
Objectives: To evaluate the safety and immunogenicity of V114 [15-valent pneumococcal conjugate vaccine (PCV) containing serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9 V, 14, 18C, 19A, 19F, 22F, 23F, 33F], followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23) 8 weeks later, in children with HIV.
Design: This phase 3 study (NCT03921424) randomized participants 6-17 years of age with HIV (CD4 + T-cell count ≥200 cells/μl, plasma HIV RNA <50 000 copies/ml) to receive V114 or 13-valent PCV (PCV13) in a double-blind manner on Day 1, followed by PPSV23 at Week 8.
Methods: Adverse events (AEs), pneumococcal serotype-specific immunoglobulin G (IgG), and opsonophagocytic activity (OPA) were evaluated 30 days after each vaccination.
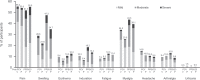
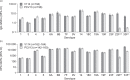
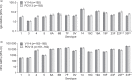
Results: The proportion of participants experiencing at least one AE post-PCV was 78.8% in the V114 group ( n = 203) and 69.6% in the PCV13 group ( n = 204); respective proportions post-PPSV23 were 75.4% ( n = 203) and 77.2% ( n = 202). There were no vaccine-related serious AEs. IgG geometric mean concentrations (GMCs) and OPA geometric mean titers (GMTs) were generally comparable between V114 and PCV13 for shared serotypes at Day 30, and were higher for V114 compared with PCV13 for the additional V114 serotypes 22F and 33F. Approximately 30 days after PPSV23, IgG GMCs and OPA GMTs were generally comparable between the V114 and PCV13 groups for all 15 serotypes in V114.
Conclusions: In children with HIV, a sequential administration of V114 followed 8 weeks later with PPSV23 is well tolerated and induces immune responses for all 15 pneumococcal serotypes included in V114.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.W., E.M., L. Morgan, K.F., and R.L. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Rahway, New Jersey, USA. J.C., L. Musey and K.B. were employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA at the time of this study.
P.K. has received a grant from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA through the university to run this study.
R.D. has received grants from Pfizer, MSD, and MedImmune/AstraZeneca. He serves as scientific consultant on the review/board/advisory committee of Pfizer and MSD. He is also part of the speakers’ bureaus of Pfizer, MSD, Sanofi Pasteur, and GlaxoSmithKline.
P.R. has participated in scientific advisory boards for Merck & Co., Pfizer and GlaxoSmithKline and has received institutional funding for investigator-initiated research from GlaxoSmithKline Biologicals and Merck & Co.
All other authors report no potential conflicts of interest.
Figures

Comment in
-
Keeping up with Streptococcus pneumoniae in children living with HIV.AIDS. 2023 Jul 1;37(8):1325-1327. doi: 10.1097/QAD.0000000000003544. AIDS. 2023. PMID: 37930313 No abstract available.
References
-
- Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis 2000; 31:170–176. - PubMed
-
- Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Moore DP, Klugman KP, et al. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS 2011; 25:453–462. - PubMed
-
- Meiring S, Cohen C, Quan V, de Gouveia L, Feldman C, Karstaedt A, et al. HIV infection and the epidemiology of invasive pneumococcal disease (IPD) in South African adults and older children prior to the introduction of a pneumococcal conjugate vaccine (PCV). PLoS One 2016; 11:e0149104. - PMC - PubMed
-
- European Centre for Disease Prevention and Control. Annual epidemiological report for 2018: invasive pneumococcal disease. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_IP... [Accessed 2 March 2022].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

