Effectiveness of Structured Care Coordination for Children With Medical Complexity: The Complex Care for Kids Ontario (CCKO) Randomized Clinical Trial
- PMID: 36939728
- PMCID: PMC10028546
- DOI: 10.1001/jamapediatrics.2023.0115
Effectiveness of Structured Care Coordination for Children With Medical Complexity: The Complex Care for Kids Ontario (CCKO) Randomized Clinical Trial
Abstract
Importance: Children with medical complexity (CMC) have chronic conditions and high health needs and may experience fragmented care.
Objective: To compare the effectiveness of a structured complex care program, Complex Care for Kids Ontario (CCKO), with usual care.
Design, setting, and participants: This randomized clinical trial used a waitlist variation for randomizing patients from 12 complex care clinics in Ontario, Canada, over 2 years. The study was conducted from December 2016 to June 2021. Participants were identified based on complex care clinic referral and randomly allocated into an intervention group, seen at the next available clinic appointment, or a control group that was placed on a waitlist to receive the intervention after 12 months.
Intervention: Assignment of a nurse practitioner-pediatrician dyad partnering with families in a structured complex care clinic to provide intensive care coordination and comprehensive plans of care.
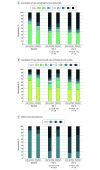
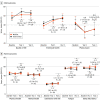
Main outcomes and measures: Co-primary outcomes, assessed at baseline and at 6, 12, and 24 months postrandomization, were service delivery indicators from the Family Experiences With Coordination of Care that scored (1) coordination of care among health care professionals, (2) coordination of care between health care professionals and families, and (3) utility of care planning tools. Secondary outcomes included child and parent health outcomes and child health care system utilization and cost.
Results: Of 144 participants randomized, 141 had complete health administrative data, and 139 had complete baseline surveys. The median (IQR) age of the participants was 29 months (9-102); 83 (60%) were male. At 12 months, scores for utility of care planning tools improved in the intervention group compared with the waitlist group (adjusted odds ratio, 9.3; 95% CI, 3.9-21.9; P < .001), with no difference between groups for the other 2 co-primary outcomes. There were no group differences for secondary outcomes of child outcomes, parent outcomes, and health care system utilization and cost. At 24 months, when both groups were receiving the intervention, no primary outcome differences were observed. Total health care costs in the second year were lower for the intervention group (median, CAD$17 891; IQR, 6098-61 346; vs CAD$37 524; IQR, 9338-119 547 [US $13 415; IQR, 4572-45 998; vs US $28 136; IQR, 7002-89 637]; P = .01).
Conclusions and relevance: The CCKO program improved the perceived utility of care planning tools but not other outcomes at 1 year. Extended evaluation periods may be helpful in assessing pediatric complex care interventions.
Trial registration: ClinicalTrials.gov Identifier: NCT02928757.
Conflict of interest statement
Figures

Comment in
-
Knowledge to Advance the Clinical Effectiveness of Pediatric Complex Care.JAMA Pediatr. 2023 May 1;177(5):453-455. doi: 10.1001/jamapediatrics.2023.0136. JAMA Pediatr. 2023. PMID: 36939710 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

