Repeat Ivermectin Mass Drug Administrations for Malaria Control II: Protocol for a Double-blind, Cluster-Randomized, Placebo-Controlled Trial for the Integrated Control of Malaria
- PMID: 36939832
- PMCID: PMC10132043
- DOI: 10.2196/41197
Repeat Ivermectin Mass Drug Administrations for Malaria Control II: Protocol for a Double-blind, Cluster-Randomized, Placebo-Controlled Trial for the Integrated Control of Malaria
Abstract
Background: The gains made against malaria have stagnated since 2015, threatened further by increasing resistance to insecticides and antimalarials. Improvement in malaria control necessitates a multipronged strategy, which includes the development of novel tools. One such tool is mass drug administration (MDA) with endectocides, primarily ivermectin, which has shown promise in reducing malaria transmission through lethal and sublethal impacts on the mosquito vector.
Objective: The primary objective of the study is to assess the impact of repeated ivermectin MDA on malaria incidence in children aged ≤10 years.
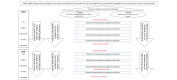
Methods: Repeat Ivermectin MDA for Malaria Control II is a double-blind, placebo-controlled, cluster-randomized, and parallel-group trial conducted in a setting with intense seasonal malaria transmission in Southwest Burkina Faso. The study included 14 discrete villages: 7 (50%) randomized to receive standard measures (seasonal malaria chemoprevention [SMC] and bed net use for children aged 3 to 59 months) and placebo, and 7 (50%) randomized to receive standard measures and monthly ivermectin MDA at 300 μg/kg for 3 consecutive days, provided under supervision to all eligible village inhabitants, over 2 successive rainy seasons. Nonpregnant individuals >90 cm in height were eligible for ivermectin MDA, and cotreatment with ivermectin and SMC was not permitted. The primary outcome is malaria incidence in children aged ≤10 years, as assessed by active case surveillance. The secondary safety outcome of repeated ivermectin MDA was assessed through active and passive adverse event monitoring.
Results: The trial intervention was conducted from July to November in 2019 and 2020, with additional sampling of humans and mosquitoes occurring through February 2022 to assess postintervention changes in transmission patterns. Additional human and entomological assessments were performed over the 2 years in a subset of households from 6 cross-sectional villages. A subset of individuals underwent additional sampling in 2020 to characterize ivermectin pharmacokinetics and pharmacodynamics. Analysis and unblinding will commence once the database has been completed, cleaned, and locked.
Conclusions: Our trial represents the first study to directly assess the impact of a novel approach for malaria control, ivermectin MDA as a mosquitocidal agent, layered into existing standard-of-care interventions. The study was designed to leverage the current SMC deployment infrastructure and will provide evidence regarding the additional benefit of ivermectin MDA in reducing malaria incidence in children.
Trial registrations: ClinicalTrials.gov NCT03967054; https://clinicaltrials.gov/ct2/show/NCT03967054 and Pan African Clinical Trials Registry PACT201907479787308; https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=8219.
International registered report identifier (irrid): DERR1-10.2196/41197.
Keywords: Anopheles; clinical trial; cluster-randomized; endectocide; ivermectin; malaria; mass drug administration; mosquito; seasonal malaria chemoprevention; transmission.
©Brian D Foy, Anthony Some, Tereza Magalhaes, Lyndsey Gray, Sangeeta Rao, Emmanuel Sougue, Conner L Jackson, John Kittelson, Hannah C Slater, Teun Bousema, Ollo Da, A Gafar V Coulidiaty, McKenzie Colt, Martina Wade, Kacey Richards, A Fabrice Some, Roch K Dabire, Sunil Parikh. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 20.03.2023.
Conflict of interest statement
Conflicts of Interest: In October 2020, BDF and SP joined the Scientific Advisory Board as consultants for the IMPACT project being run by MedinCell. RKD and AFS are investigators for the IMPACT project. BDF also performed a 1-time consultation for Lyndra Therapeutics Inc in July 2020.
Figures


References
-
- Slater HC, Foy BD, Kobylinski K, Chaccour C, Watson OJ, Hellewell J, Aljayyoussi G, Bousema T, Burrows J, D'Alessandro U, Alout H, Ter Kuile FO, Walker PG, Ghani AC, Smit MR. Ivermectin as a novel complementary malaria control tool to reduce incidence and prevalence: a modelling study. Lancet Infect Dis. 2020 Apr;20(4):498–508. doi: 10.1016/S1473-3099(19)30633-4. https://hal.archives-ouvertes.fr/hal-02450316 S1473-3099(19)30633-4 - DOI - PubMed
-
- WHO guidelines for malaria, 3 June 2022. World Health Organization. 2022. [2023-02-27]. https://apps.who.int/iris/handle/10665/354781 .
-
- World Malaria Report 2021. Geneva: World Health Organization; 2021.
-
- Chaccour CJ, Kobylinski KC, Bassat Q, Bousema T, Drakeley C, Alonso P, Foy BD. Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J. 2013 May 07;12:153. doi: 10.1186/1475-2875-12-153. https://malariajournal.biomedcentral.com/articles/10.1186/1475-2875-12-153 1475-2875-12-153 - DOI - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

