Biomarkers and cardiovascular outcomes in chimeric antigen receptor T-cell therapy recipients
- PMID: 36939851
- PMCID: PMC10256191
- DOI: 10.1093/eurheartj/ehad117
Biomarkers and cardiovascular outcomes in chimeric antigen receptor T-cell therapy recipients
Abstract
Aims: Chimeric antigen receptor T-cell therapy (CAR-T) harnesses a patient's immune system to target cancer. There are sparse existing data characterizing death outcomes after CAR-T-related cardiotoxicity. This study examines the association between CAR-T-related severe cardiovascular events (SCE) and mortality.
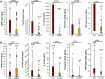
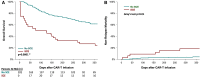
Methods and results: From a multi-centre registry of 202 patients receiving anti-CD19 CAR-T, covariates including standard baseline cardiovascular and cancer parameters and biomarkers were collected. Severe cardiovascular events were defined as a composite of heart failure, cardiogenic shock, or myocardial infarction. Thirty-three patients experienced SCE, and 108 patients died during a median follow-up of 297 (interquartile range 104-647) days. Those that did and did not die after CAR-T were similar in age, sex, and prior anthracycline use. Those who died had higher peak interleukin (IL)-6 and ferritin levels after CAR-T infusion, and those who experienced SCE had higher peak IL-6, C-reactive protein (CRP), ferritin, and troponin levels. The day-100 and 1-year Kaplan-Meier overall mortality estimates were 18% and 43%, respectively, while the non-relapse mortality (NRM) cumulative incidence rates were 3.5% and 6.7%, respectively. In a Cox model, SCE occurrence following CAR-T was independently associated with increased overall mortality risk [hazard ratio (HR) 2.8, 95% confidence interval (CI) 1.6-4.7] after adjusting for age, cancer type and burden, anthracycline use, cytokine release syndrome grade ≥ 2, pre-existing heart failure, hypertension, and African American ancestry; SCEs were independently associated with increased NRM (HR 3.5, 95% CI 1.4-8.8) after adjusting for cancer burden.
Conclusion: Chimeric antigen receptor T-cell therapy recipients who experience SCE have higher overall mortality and NRM and higher peak levels of IL-6, CRP, ferritin, and troponin.
Keywords: CAR-T cells; Cancer; Cardio-oncology; Cardiovascular events; Chimeric antigen receptor; Mortality.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest S.S.M. has received consulting fees from Nektar Therapeutics, Health & Wellness Partners, Medicure. P.A.R. has received consulting fees from AbbVie, BMS, Janssen, Novartis, BeiGene, Kite/Gilead, Intellia Therapeutics, Sana Biotechnology, CVS Caremark, Genmab, Pharmacyclics, Takeda, Karyopharm, Nektar Therapeutics, Nurix Therapeutics, and ADC Therapeutics. M.A.P. reports consulting fees from Adicet, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma, is participating in DSMB of Cidara Therapeutics, Medigene, and Sellas Life Sciences, and is on the scientific advisory board of NexImmune and Omeros. M.B.G. has received institutional grant funding from Sanofi, Amgen, and Actinium and consulting fees from Sanofi, Novartis, and Allogene. M.L.P. has received royalties from Juno and Sers and consulting fees from Novartis, Cellectar, Synthekine, Kite, Seres, Magenta, WindMIL, Rheos, Nektar, Notch, Priothera, Ceramedix, Lygenesis, and Pluto. R.S. has received consulting fees from Mudexus and MyBiotics. G.S. has received research funding from Janssen, Amgen, Beyond Spring, and BMS and is on DSMB of ArcellX. E.H.Y. has received institutional grand funding from CSL Behring, Boehringer Ingelheim, BMS and Eli and Lilly and consulting fees from Pfizer. J.P.L. has received institutional grants from Genentech, Janssen, and Epizyme and consulting fees from Abbvie, Astellas, AstraZeneca, Bayer, Beigene, BMS, Calithera, Constellation, Caribou Biosciences, Eisai, Lilly, Epizyme, Genmab, Grail, Incyte, Jansssen, MEI Pharma, Merck, Mustang Bio, Novartis, Pfizer, Roche/Genentech, Seagen, Second Genome, Sutro, ADC Therapeutics, Miltenyi, and Karyopharm. T.G.N. has received consulting fees from BMS, Genentech, Abbvie, Roche, CRO Oncology, and Sanofi and participates in DSMB of Genentech and received research grant funding from AstraZeneca and BMS.
Figures


Comment in
-
CAR T-cell cancer therapies: do not forget the heart.Eur Heart J. 2023 Jun 9;44(22):2043-2045. doi: 10.1093/eurheartj/ehad175. Eur Heart J. 2023. PMID: 36994886 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

