Atezolizumab Plus PEGPH20 Versus Chemotherapy in Advanced Pancreatic Ductal Adenocarcinoma and Gastric Cancer: MORPHEUS Phase Ib/II Umbrella Randomized Study Platform
- PMID: 36940261
- PMCID: PMC10243783
- DOI: 10.1093/oncolo/oyad022
Atezolizumab Plus PEGPH20 Versus Chemotherapy in Advanced Pancreatic Ductal Adenocarcinoma and Gastric Cancer: MORPHEUS Phase Ib/II Umbrella Randomized Study Platform
Abstract
Background: The MORPHEUS platform comprises multiple open-label, randomized, phase Ib/II trials designed to identify early efficacy and safety signals of treatment combinations across cancers. Atezolizumab (anti-programmed cell death 1 ligand 1 [PD-L1]) was evaluated in combination with PEGylated recombinant human hyaluronidase (PEGPH20).
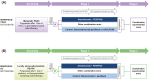
Methods: In 2 randomized MORPHEUS trials, eligible patients with advanced, previously treated pancreatic ductal adenocarcinoma (PDAC) or gastric cancer (GC) received atezolizumab plus PEGPH20, or control treatment (mFOLFOX6 or gemcitabine plus nab-paclitaxel [MORPHEUS-PDAC]; ramucirumab plus paclitaxel [MORPHEUS-GC]). Primary endpoints were objective response rates (ORR) per RECIST 1.1 and safety.
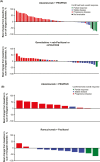
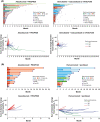
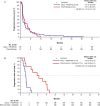
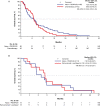
Results: In MORPHEUS-PDAC, ORRs with atezolizumab plus PEGPH20 (n = 66) were 6.1% (95% CI, 1.68%-14.80%) vs. 2.4% (95% CI, 0.06%-12.57%) with chemotherapy (n = 42). In the respective arms, 65.2% and 61.9% had grade 3/4 adverse events (AEs); 4.5% and 2.4% had grade 5 AEs. In MORPHEUS-GC, confirmed ORRs with atezolizumab plus PEGPH20 (n = 13) were 0% (95% CI, 0%-24.7%) vs. 16.7% (95% CI, 2.1%-48.4%) with control (n = 12). Grade 3/4 AEs occurred in 30.8% and 75.0% of patients, respectively; no grade 5 AEs occurred.
Conclusion: Atezolizumab plus PEGPH20 showed limited clinical activity in patients with PDAC and none in patients with GC. The safety of atezolizumab plus PEGPH20 was consistent with each agent's known safety profile. (ClinicalTrials.gov Identifier: NCT03193190 and NCT03281369).
Keywords: PD-L1; basket study; combination therapy; gastric cancer; hyaluronan; immunotherapy; pancreatic cancer; proof of concept.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
All authors received support from F. Hoffmann-La Roche/Genentech during the conduct of this study.
Figures

References
-
- Hingorani SR, Zheng L, Bullock AJ, et al. . HALO 202: Randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36(4):359-366. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

