Effect of Thromboprophylaxis on Clinical Outcomes After COVID-19 Hospitalization
- PMID: 36940444
- PMCID: PMC10064277
- DOI: 10.7326/M22-3350
Effect of Thromboprophylaxis on Clinical Outcomes After COVID-19 Hospitalization
Abstract
Background: Patients hospitalized with COVID-19 have an increased incidence of thromboembolism. The role of extended thromboprophylaxis after hospital discharge is unclear.
Objective: To determine whether anticoagulation is superior to placebo in reducing death and thromboembolic complications among patients discharged after COVID-19 hospitalization.
Design: Prospective, randomized, double-blind, placebo-controlled clinical trial. (ClinicalTrials.gov: NCT04650087).
Setting: Done during 2021 to 2022 among 127 U.S. hospitals.
Participants: Adults aged 18 years or older hospitalized with COVID-19 for 48 hours or more and ready for discharge, excluding those with a requirement for, or contraindication to, anticoagulation.
Intervention: 2.5 mg of apixaban versus placebo twice daily for 30 days.
Measurements: The primary efficacy end point was a 30-day composite of death, arterial thromboembolism, and venous thromboembolism. The primary safety end points were 30-day major bleeding and clinically relevant nonmajor bleeding.
Results: Enrollment was terminated early, after 1217 participants were randomly assigned, because of a lower than anticipated event rate and a declining rate of COVID-19 hospitalizations. Median age was 54 years, 50.4% were women, 26.5% were Black, and 16.7% were Hispanic; 30.7% had a World Health Organization severity score of 5 or greater, and 11.0% had an International Medical Prevention Registry on Venous Thromboembolism risk prediction score of greater than 4. Incidence of the primary end point was 2.13% (95% CI, 1.14 to 3.62) in the apixaban group and 2.31% (CI, 1.27 to 3.84) in the placebo group. Major bleeding occurred in 2 (0.4%) and 1 (0.2%) and clinically relevant nonmajor bleeding occurred in 3 (0.6%) and 6 (1.1%) apixaban-treated and placebo-treated participants, respectively. By day 30, thirty-six (3.0%) participants were lost to follow-up, and 8.5% of apixaban and 11.9% of placebo participants permanently discontinued the study drug treatment.
Limitations: The introduction of SARS-CoV-2 vaccines decreased the risk for hospitalization and death. Study enrollment spanned the peaks of the Delta and Omicron variants in the United States, which influenced illness severity.
Conclusion: The incidence of death or thromboembolism was low in this cohort of patients discharged after hospitalization with COVID-19. Because of early enrollment termination, the results were imprecise and the study was inconclusive.
Primary funding source: National Institutes of Health.
Conflict of interest statement
Figures

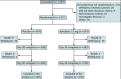
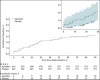

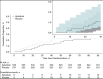
Comment in
-
Seeing the Positive in Negative Studies.Ann Intern Med. 2023 Apr;176(4):561-562. doi: 10.7326/M23-0576. Epub 2023 Mar 21. Ann Intern Med. 2023. PMID: 36940441 No abstract available.
References
-
- Cuker A , Tseng EK , Schünemann HJ , et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis for patients with COVID-19: March 2022 update on the use of anticoagulation in critically ill patients. Blood Adv. 2022;6:4975-82. [PMID: ] doi: 10.1182/bloodadvances.2022007940 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
