Induction of the alternative lengthening of telomeres pathway by trapping of proteins on DNA
- PMID: 36940725
- PMCID: PMC10359465
- DOI: 10.1093/nar/gkad150
Induction of the alternative lengthening of telomeres pathway by trapping of proteins on DNA
Abstract
Telomere maintenance is a hallmark of malignant cells and allows cancers to divide indefinitely. In some cancers, this is achieved through the alternative lengthening of telomeres (ALT) pathway. Whilst loss of ATRX is a near universal feature of ALT-cancers, it is insufficient in isolation. As such, other cellular events must be necessary - but the exact nature of the secondary events has remained elusive. Here, we report that trapping of proteins (such as TOP1, TOP2A and PARP1) on DNA leads to ALT induction in cells lacking ATRX. We demonstrate that protein-trapping chemotherapeutic agents, such as etoposide, camptothecin and talazoparib, induce ALT markers specifically in ATRX-null cells. Further, we show that treatment with G4-stabilising drugs cause an increase in trapped TOP2A levels which leads to ALT induction in ATRX-null cells. This process is MUS81-endonuclease and break-induced replication dependent, suggesting that protein trapping leads to replication fork stalling, with these forks being aberrantly processed in the absence of ATRX. Finally, we show ALT-positive cells harbour a higher load of genome-wide trapped proteins, such as TOP1, and knockdown of TOP1 reduced ALT activity. Taken together, these findings suggest that protein trapping is a fundamental driving force behind ALT-biology in ATRX-deficient malignancies.
Plain language summary
A key feature of all cancer cells is their ability to divide indefinitely, and this is dependent on circumvention of telomere shortening through induction of a telomere maintenance mechanism, such as the telomerase-independent, Alternative Lengthening of Telomeres (ALT) pathway. The ALT pathway is characterised by loss of the ATRX chromatin remodeler. The current study provides evidence that, in the absence of ATRX, increased trapping of proteins on DNA leads to replication fork stalling and collapse. At telomeres, this leads to ALT pathway activity. These results help to better understand ALT tumours and might, eventually, be instrumental in developing new therapeutic strategies.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

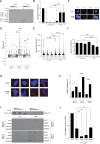

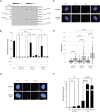
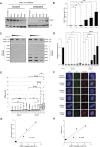


References
-
- de Lange T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018; 52:223–247. - PubMed
-
- Heaphy C.M., Subhawong A.P., Hong S.M., Goggins M.G., Montgomery E.A., Gabrielson E., Netto G.J., Epstein J.I., Lotan T.L., Westra W.H.et al. .. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol. 2011; 179:1608–1615. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

