Therapeutic potential of Clostridium butyricum anticancer effects in colorectal cancer
- PMID: 36941257
- PMCID: PMC10038047
- DOI: 10.1080/19490976.2023.2186114
Therapeutic potential of Clostridium butyricum anticancer effects in colorectal cancer
Abstract
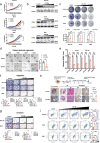
Probiotic roles of Clostridium butyricum (C.B) are involved in regulating disease and cancers, yet the mechanistic basis for these regulatory roles remains largely unknown. Here, we demonstrate that C.B reprograms the proliferation, migration, stemness, and tumor growth in CRC by regulating pivotal signal molecules including MYC. Destabilization of MYC by C.B supplementation suppresses cancer cell proliferation/metastasis, sensitizes 5-FU treatment, and boosts responsiveness of anti-PD1 therapy. MYC is a transcriptional regulator of Thymidylate synthase (TYMS), a key target of the 5-FU. Also MYC is known to impact on PD-1 expression. Mechanistically, C.B treatment of CRC cells results in MYC degradation by enhancing proteasome-mediated ubiquitination, thereby mitigating MYC-mediated 5-FU resistance and boosting anti-PD1 immunotherapeutic efficacy. Together, our findings uncover previously unappreciated links between C.B and CRC cell signaling, providing insight into the tumorigenesis modulating mechanisms of C.B in boosting chemo/immune therapies.
Keywords: 5-FU; Anti-PD1; Clostridium butyricum; MYC; TYMS; immunotherapy; ubiquitination.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
