Hydrogels for RNA delivery
- PMID: 36941391
- PMCID: PMC10330049
- DOI: 10.1038/s41563-023-01472-w
Hydrogels for RNA delivery
Abstract
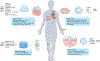
RNA-based therapeutics have shown tremendous promise in disease intervention at the genetic level, and some have been approved for clinical use, including the recent COVID-19 messenger RNA vaccines. The clinical success of RNA therapy is largely dependent on the use of chemical modification, ligand conjugation or non-viral nanoparticles to improve RNA stability and facilitate intracellular delivery. Unlike molecular-level or nanoscale approaches, macroscopic hydrogels are soft, water-swollen three-dimensional structures that possess remarkable features such as biodegradability, tunable physiochemical properties and injectability, and recently they have attracted enormous attention for use in RNA therapy. Specifically, hydrogels can be engineered to exert precise spatiotemporal control over the release of RNA therapeutics, potentially minimizing systemic toxicity and enhancing in vivo efficacy. This Review provides a comprehensive overview of hydrogel loading of RNAs and hydrogel design for controlled release, highlights their biomedical applications and offers our perspectives on the opportunities and challenges in this exciting field of RNA delivery.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
R.L. declares the following financial interests: Alnylam Pharmaceuticals, Inc. and Moderna, Inc. For a list of entities with which R.L. is involved, compensated or uncompensated, see the Supplementary Note. J.C. is a co-founder and shareholder of TargTex S.A. - Targeted Therapeutics for Glioblastoma Multiforme. The other authors declare no competing interests.
Figures
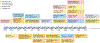
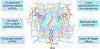



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

