Campylobacter jejuni: targeting host cells, adhesion, invasion, and survival
- PMID: 36941439
- PMCID: PMC10027602
- DOI: 10.1007/s00253-023-12456-w
Campylobacter jejuni: targeting host cells, adhesion, invasion, and survival
Abstract
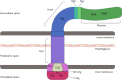
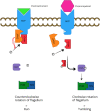
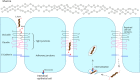
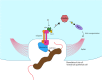
Campylobacter jejuni, causing strong enteritis, is an unusual bacterium with numerous peculiarities. Chemotactically controlled motility in viscous milieu allows targeted navigation to intestinal mucus and colonization. By phase variation, quorum sensing, extensive O-and N-glycosylation and use of the flagellum as type-3-secretion system C. jejuni adapts effectively to environmental conditions. C. jejuni utilizes proteases to open cell-cell junctions and subsequently transmigrates paracellularly. Fibronectin at the basolateral side of polarized epithelial cells serves as binding site for adhesins CadF and FlpA, leading to intracellular signaling, which again triggers membrane ruffling and reduced host cell migration by focal adhesion. Cell contacts of C. jejuni results in its secretion of invasion antigens, which induce membrane ruffling by paxillin-independent pathway. In addition to fibronectin-binding proteins, other adhesins with other target structures and lectins and their corresponding sugar structures are involved in host-pathogen interaction. Invasion into the intestinal epithelial cell depends on host cell structures. Fibronectin, clathrin, and dynein influence cytoskeletal restructuring, endocytosis, and vesicular transport, through different mechanisms. C. jejuni can persist over a 72-h period in the cell. Campylobacter-containing vacuoles, avoid fusion with lysosomes and enter the perinuclear space via dynein, inducing signaling pathways. Secretion of cytolethal distending toxin directs the cell into programmed cell death, including the pyroptotic release of proinflammatory substances from the destroyed cell compartments. The immune system reacts with an inflammatory cascade by participation of numerous immune cells. The development of autoantibodies, directed not only against lipooligosaccharides, but also against endogenous gangliosides, triggers autoimmune diseases. Lesions of the epithelium result in loss of electrolytes, water, and blood, leading to diarrhea, which flushes out mucus containing C. jejuni. Together with the response of the immune system, this limits infection time. Based on the structural interactions between host cell and bacterium, the numerous virulence mechanisms, signaling, and effects that characterize the infection process of C. jejuni, a wide variety of targets for attenuation of the pathogen can be characterized. The review summarizes strategies of C. jejuni for host-pathogen interaction and should stimulate innovative research towards improved definition of targets for future drug development. KEY POINTS: • Bacterial adhesion of Campylobacter to host cells and invasion into host cells are strictly coordinated processes, which can serve as targets to prevent infection. • Reaction and signalling of host cell depend on the cell type. • Campylobacter virulence factors can be used as targets for development of antivirulence drug compounds.
Keywords: Adhesion; Antiadhesion; Campylobacter; Host–pathogen interaction; Invasion; Virulence factors.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Akhondi H, Simonsen KA. Bacterial diarrhea. Treasure Island (FL): StatPearls Publishing; 2022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

