Whole-body vibration ameliorates glial pathological changes in the hippocampus of hAPP transgenic mice, but does not affect plaque load
- PMID: 36941713
- PMCID: PMC10026461
- DOI: 10.1186/s12993-023-00208-9
Whole-body vibration ameliorates glial pathological changes in the hippocampus of hAPP transgenic mice, but does not affect plaque load
Abstract
Background: Alzheimer's disease (AD) is the core cause of dementia in elderly populations. One of the main hallmarks of AD is extracellular amyloid beta (Aβ) accumulation (APP-pathology) associated with glial-mediated neuroinflammation. Whole-Body Vibration (WBV) is a passive form of exercise, but its effects on AD pathology are still unknown.
Methods: Five months old male J20 mice (n = 26) and their wild type (WT) littermates (n = 24) were used to investigate the effect of WBV on amyloid pathology and the healthy brain. Both J20 and WT mice underwent WBV on a vibration platform or pseudo vibration treatment. The vibration intervention consisted of 2 WBV sessions of 10 min per day, five days per week for five consecutive weeks. After five weeks of WBV, the balance beam test was used to assess motor performance. Brain tissue was collected to quantify Aβ deposition and immunomarkers of astrocytes and microglia.
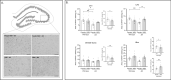
Results: J20 mice have a limited number of plaques at this relatively young age. Amyloid plaque load was not affected by WBV. Microglia activation based on IBA1-immunostaining was significantly increased in the J20 animals compared to the WT littermates, whereas CD68 expression was not significantly altered. WBV treatment was effective to ameliorate microglia activation based on morphology in both J20 and WT animals in the Dentate Gyrus, but not so in the other subregions. Furthermore, GFAP expression based on coverage was reduced in J20 pseudo-treated mice compared to the WT littermates and it was significantly reserved in the J20 WBV vs. pseudo-treated animals. Further, only for the WT animals a tendency of improved motor performance was observed in the WBV group compared to the pseudo vibration group.
Conclusion: In accordance with the literature, we detected an early plaque load, reduced GFAP expression and increased microglia activity in J20 mice at the age of ~ 6 months. Our findings indicate that WBV has beneficial effects on the early progression of brain pathology. WBV restored, above all, the morphology of GFAP positive astrocytes to the WT level that could be considered the non-pathological and hence "healthy" level. Next experiments need to be performed to determine whether WBV is also affective in J20 mice of older age or other AD mouse models.
Keywords: Amyloid beta; J20 mice; Motor coordination; Neuroinflammation; Passive exercise.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

