Coverage of primary and booster vaccination against COVID-19 by socioeconomic level: A nationwide cross-sectional registry study
- PMID: 36941785
- PMCID: PMC10072069
- DOI: 10.1080/21645515.2023.2188857
Coverage of primary and booster vaccination against COVID-19 by socioeconomic level: A nationwide cross-sectional registry study
Abstract
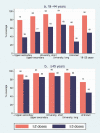
High and equitable COVID-19 vaccination coverage is important for pandemic control and prevention of health inequity. However, little is known about socioeconomic correlates of booster vaccination coverage. In this cross-sectional study of all Norwegian adults in the national vaccination program (N = 4,190,655), we use individual-level registry data to examine coverage by levels of household income and education of primary (≥2 doses) and booster (≥3 doses) vaccination against COVID-19. We stratify the analyses by age groups with different booster recommendations and report relative risk ratios (RR) for vaccination by 25 August 2022. In the 18-44 y group, individuals with highest vs. lowest education had 94% vs. 79% primary coverage (adjusted RR (adjRR) 1.15, 95%CI 1.14-1.15) and 67% vs. 38% booster coverage (adjRR 1.55, 95% CI 1.55-1.56), while individuals with highest vs. lowest income had 94% vs. 81% primary coverage (adjRR 1.10, 95%CI 1.10-1.10) and 60% vs. 43% booster coverage (adjRR 1.23, 95%CI 1.22-1.24). In the ≥45 y group, individuals with highest vs. lowest education had 96% vs. 92% primary coverage (adjRR 1.02, 95%CI 1.02-1.02) and 88% vs. 80% booster coverage (adjRR 1.09, 95%CI 1.09-1.09), while individuals with highest vs. lowest income had 98% vs. 82% primary coverage (adjRR 1.16, 95%CI 1.16-1.16) and 92% vs. 64% booster coverage (adjRR 1.33, 95%CI 1.33-1.34). In conclusion, we document large socioeconomic inequalities in COVID-19 vaccination coverage, especially for booster vaccination, even though all vaccination was free-of-charge. The results highlight the need to tailor information and to target underserved groups for booster vaccination.
Keywords: Booster vaccine; COVID-19; disparity; social inequity; sociodemographic correlates; socioeconomic correlates; vaccination program; vaccine coverage; vaccine hesitancy; vaccine uptake.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Inequities in COVID-19 vaccine and booster coverage across Massachusetts ZIP codes after the emergence of Omicron: A population-based cross-sectional study.PLoS Med. 2023 Jan 31;20(1):e1004167. doi: 10.1371/journal.pmed.1004167. eCollection 2023 Jan. PLoS Med. 2023. PMID: 36719864 Free PMC article.
-
Geographic, Occupational, and Sociodemographic Variations in Uptake of COVID-19 Booster Doses Among Fully Vaccinated US Adults, December 1, 2021, to January 10, 2022.JAMA Netw Open. 2022 Aug 1;5(8):e2227680. doi: 10.1001/jamanetworkopen.2022.27680. JAMA Netw Open. 2022. PMID: 35984657 Free PMC article.
-
Uncovering inequities in Covid-19 vaccine coverage for adults and elderly in Brazil: A multilevel study of 2021-2022 data.Vaccine. 2023 Jun 13;41(26):3937-3945. doi: 10.1016/j.vaccine.2023.05.030. Epub 2023 May 17. Vaccine. 2023. PMID: 37221119 Free PMC article.
-
Acceptance of COVID-19 Vaccine Booster Doses Using the Health Belief Model: A Cross-Sectional Study in Low-Middle- and High-Income Countries of the East Mediterranean Region.Int J Environ Res Public Health. 2022 Sep 25;19(19):12136. doi: 10.3390/ijerph191912136. Int J Environ Res Public Health. 2022. PMID: 36231447 Free PMC article.
-
Global diversity of policy, coverage, and demand of COVID-19 vaccines: a descriptive study.BMC Med. 2022 Apr 4;20(1):130. doi: 10.1186/s12916-022-02333-0. BMC Med. 2022. PMID: 35369871 Free PMC article.
Cited by
-
The Potential for Twice-Annual Influenza Vaccination to Reduce Disease Burden.Influenza Other Respir Viruses. 2025 Mar;19(3):e70052. doi: 10.1111/irv.70052. Influenza Other Respir Viruses. 2025. PMID: 40045876 Free PMC article.
-
Factors underlying COVID-19 booster vaccine uptake among adults in Belgium.BMC Res Notes. 2023 Nov 11;16(1):328. doi: 10.1186/s13104-023-06608-4. BMC Res Notes. 2023. PMID: 37951923 Free PMC article.
-
Perceptions of childhood immunization in São Paulo: quantitative-qualitative cross-sectional study.Sao Paulo Med J. 2024 Oct 21;142(6):e2023447. doi: 10.1590/1516-3180.2023.0447.R1.05062024. eCollection 2024. Sao Paulo Med J. 2024. PMID: 39442092 Free PMC article.
-
COVID-19 vaccine uptake in Skåne county, Sweden, in relation to individual-level and area-level sociodemographic factors: a register-based cross-sectional analysis.BMJ Public Health. 2024 Mar 25;2(1):e000437. doi: 10.1136/bmjph-2023-000437. eCollection 2024 Jun. BMJ Public Health. 2024. PMID: 40018154 Free PMC article.
-
COVID-19 vaccine uptake in a predominantly minoritized cohort hospitalized during the early pandemic in New York City.Vaccine. 2024 Dec 2;42(26):126260. doi: 10.1016/j.vaccine.2024.126260. Epub 2024 Sep 11. Vaccine. 2024. PMID: 39265456
References
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis, E, Singer, SR, Khan, F, Brooks, N, Smaja, M, Mircus, G, Pan, K, Southern, J.. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–10. doi:10.1016/S0140-6736(21)00947-8. - DOI - PMC - PubMed
-
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, Brown K.. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi:10.1136/bmj.n1088. - DOI - PMC - PubMed
-
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG, Wilder-Smith A. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44. doi:10.1016/S0140-6736(22)00152-0. - DOI - PMC - PubMed
-
- Gram MA, Emborg HD, Schelde AB, Friis NU, Nielsen KF, Moustsen-Helms IR, Legarth R, Lam JU, Chaine M, Malik AZ, Rasmussen M. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: a nationwide Danish cohort study. PLoS Med. 2022;19(9):e1003992. doi:10.1371/journal.pmed.1003992. - DOI - PMC - PubMed
-
- Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, Dodd K, Enever Y. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–76. doi:10.1016/S0140-6736(21)02717-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
