The Safety and Efficacy of Mesenchymal Stem Cells in the Treatment of Type 2 Diabetes- A Literature Review
- PMID: 36941907
- PMCID: PMC10024492
- DOI: 10.2147/DMSO.S392161
The Safety and Efficacy of Mesenchymal Stem Cells in the Treatment of Type 2 Diabetes- A Literature Review
Abstract
Introduction: Type 2 diabetes (T2D) is the most common type of diabetes, affecting 6.28% of the population worldwide. Over the decades, multiple therapies and drugs have been developed to control T2D, but they are far from a long-term solution. Stem cells are promising as novel regenerative treatments, especially mesenchymal stem cells (MSCs), which are highly versatile in their regenerative and paracrine capabilities and characteristics. This makes them the most commonly used adult stem cells and ideal candidates to treat diabetes.
Objective: To assess the safety and efficacy of mesenchymal stem cells (MSCs) in treating Type 2 diabetes (T2D) in humans.
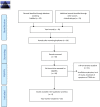
Methods: Mesenchymal stem cell-based treatments were studied in 262 patients. A total of 6 out of 58 trials fit our inclusion criteria in the last five years.
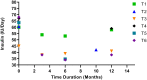
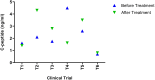
Results: The treatment of patients with MSCs reduced the dosage of anti-diabetic drugs analyzed over a follow-up period of 12 months. The effective therapy dosage ranged from 1×106 cells/kg to 3.7×106 cells/kg. After treatment, HbAc1 levels were reduced by an average of 32%, and the fasting blood glucose levels were reduced to an average of 45%. The C-peptide levels were decreased by an average of 38% in 2 trials and increased by 36% in 4 trials. No severe adverse events were noted in all trials.
Conclusion: This analysis concludes that MSC treatment of type 2 diabetes is safe and effective. A larger sample size is required, and the trials should also study the effect of differentiated MSCs as insulin-producing cells.
Keywords: diabetes; mesenchymal stem cells; regenerative medicine; stem cell.
© 2023 Mathur et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- IDF Diabetes Atlas. Diabetes facts & figures; 2021.
-
- Samar Hafida M. Type 2 Diabetes: Which Medication is Best for Me? Harvard health publishing; 2020.
Publication types
LinkOut - more resources
Full Text Sources

