Dual-bionic regenerative microenvironment for peripheral nerve repair
- PMID: 36942011
- PMCID: PMC10024190
- DOI: 10.1016/j.bioactmat.2023.02.002
Dual-bionic regenerative microenvironment for peripheral nerve repair
Abstract
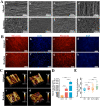
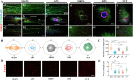
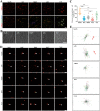
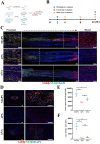
Autologous nerve grafting serves is considered the gold standard treatment for peripheral nerve defects; however, limited availability and donor area destruction restrict its widespread clinical application. Although the performance of allogeneic decellularized nerve implants has been explored, challenges such as insufficient human donors have been a major drawback to its clinical use. Tissue-engineered neural regeneration materials have been developed over the years, and researchers have explored strategies to mimic the peripheral neural microenvironment during the design of nerve catheter grafts, namely the extracellular matrix (ECM), which includes mechanical, physical, and biochemical signals that support nerve regeneration. In this study, polycaprolactone/silk fibroin (PCL/SF)-aligned electrospun material was modified with ECM derived from human umbilical cord mesenchymal stem cells (hUMSCs), and a dual-bionic nerve regeneration material was successfully fabricated. The results indicated that the developed biomimetic material had excellent biological properties, providing sufficient anchorage for Schwann cells and subsequent axon regeneration and angiogenesis processes. Moreover, the dual-bionic material exerted a similar effect to that of autologous nerve transplantation in bridging peripheral nerve defects in rats. In conclusion, this study provides a new concept for designing neural regeneration materials, and the prepared dual-bionic repair materials have excellent auxiliary regenerative ability and further preclinical testing is warranted to evaluate its clinical application potential.
Keywords: Electrospun; Extracellular matrix; Peripheral nerve regeneration; Tissue engineering; Umbilical cord mesenchymal stem cells.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hussey G.S., Dziki J.L., Badylak S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018;3(7):159–173.
-
- Scheib J., Höke A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013;9(12):668–676. - PubMed
-
- Gu X., Ding F., Williams D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35(24):6143–6156. - PubMed
LinkOut - more resources
Full Text Sources

