Neuronal guidance genes in health and diseases
- PMID: 36942388
- PMCID: PMC10121128
- DOI: 10.1093/procel/pwac030
Neuronal guidance genes in health and diseases
Abstract
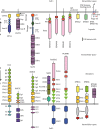
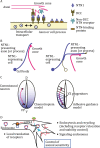
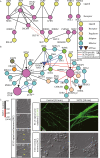
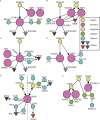
Neurons migrate from their birthplaces to the destinations, and extending axons navigate to their synaptic targets by sensing various extracellular cues in spatiotemporally controlled manners. These evolutionally conserved guidance cues and their receptors regulate multiple aspects of neural development to establish the highly complex nervous system by mediating both short- and long-range cell-cell communications. Neuronal guidance genes (encoding cues, receptors, or downstream signal transducers) are critical not only for development of the nervous system but also for synaptic maintenance, remodeling, and function in the adult brain. One emerging theme is the combinatorial and complementary functions of relatively limited classes of neuronal guidance genes in multiple processes, including neuronal migration, axonal guidance, synaptogenesis, and circuit formation. Importantly, neuronal guidance genes also regulate cell migration and cell-cell communications outside the nervous system. We are just beginning to understand how cells integrate multiple guidance and adhesion signaling inputs to determine overall cellular/subcellular behavior and how aberrant guidance signaling in various cell types contributes to diverse human diseases, ranging from developmental, neuropsychiatric, and neurodegenerative disorders to cancer metastasis. We review classic studies and recent advances in understanding signaling mechanisms of the guidance genes as well as their roles in human diseases. Furthermore, we discuss the remaining challenges and therapeutic potentials of modulating neuronal guidance pathways in neural repair.
Keywords: angiogenesis; axon guidance; cancer metastasis; cell-cell communications; neural circuit formation; neural mapping; neuronal migration; organogenesis; synaptogenesis.
©The Author(s) 2022. Published by Oxford University Press on behalf of Higher Education Press.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

