CAF-1 deposits newly synthesized histones during DNA replication using distinct mechanisms on the leading and lagging strands
- PMID: 36942484
- PMCID: PMC10164577
- DOI: 10.1093/nar/gkad171
CAF-1 deposits newly synthesized histones during DNA replication using distinct mechanisms on the leading and lagging strands
Abstract
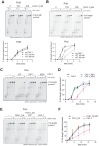
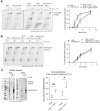
During every cell cycle, both the genome and the associated chromatin must be accurately replicated. Chromatin Assembly Factor-1 (CAF-1) is a key regulator of chromatin replication, but how CAF-1 functions in relation to the DNA replication machinery is unknown. Here, we reveal that this crosstalk differs between the leading and lagging strand at replication forks. Using biochemical reconstitutions, we show that DNA and histones promote CAF-1 recruitment to its binding partner PCNA and reveal that two CAF-1 complexes are required for efficient nucleosome assembly under these conditions. Remarkably, in the context of the replisome, CAF-1 competes with the leading strand DNA polymerase epsilon (Polϵ) for PCNA binding. However, CAF-1 does not affect the activity of the lagging strand DNA polymerase Delta (Polδ). Yet, in cells, CAF-1 deposits newly synthesized histones equally on both daughter strands. Thus, on the leading strand, chromatin assembly by CAF-1 cannot occur simultaneously to DNA synthesis, while on the lagging strand these processes may be coupled. We propose that these differences may facilitate distinct parental histone recycling mechanisms and accommodate the inherent asymmetry of DNA replication.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Bellelli R., Boulton S.J.. Spotlight on the replisome: aetiology of DNA replication-associated genetic diseases. Trends Genet. 2021; 37:317–336. - PubMed
-
- Hills S.A., Diffley J.F.X.. DNA replication and oncogene-induced replicative stress. Curr. Biol. 2014; 24:R435–R444. - PubMed
-
- Stewart-Morgan K.R., Petryk N., Groth A.. Chromatin replication and epigenetic cell memory. Nat. Cell Biol. 2020; 22:361–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

