Effects of memantine on mania-like phenotypes exhibited by Drosophila Shaker mutants
- PMID: 36942502
- PMCID: PMC10324369
- DOI: 10.1111/cns.14145
Effects of memantine on mania-like phenotypes exhibited by Drosophila Shaker mutants
Abstract
Introduction: Increased glutamate levels and electrolytic fluctuations have been observed in acutely manic patients. Despite some efficacy of the non-competitive NMDA receptor antagonist memantine (Mem), such as antidepressant-like and mood-stabilizer drugs in clinical studies, its specific mechanisms of action are still uncertain. The present study aims to better characterize the Drosophila melanogaster fly Shaker mutants (SH), as a translational model of manic episodes within bipolar disorder in humans, and to investigate the potential anti-manic properties of Mem.
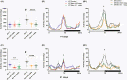
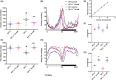
Methods and results: Our findings showed typical behavioral abnormalities in SH, which mirrored with the overexpression of NMDAR-NR1 protein subunit, matched well to glutamate up-regulation. Such molecular features were associated to a significant reduction of SH brain volume in comparison to Wild Type strain flies (WT). Here we report on the ability of Mem treatment to ameliorate behavioral aberrations of SH (similar to that of Lithium), and its ability to reduce NMDAR-NR1 over-expression.
Conclusions: Our results show the involvement of the glutamatergic system in the SH, given the interaction between the Shaker channel and the NMDA receptor, suggesting this model as a promising tool for studying the neurobiology of bipolar disorders. Moreover, our results show Mem as a potential disease-modifying therapy, providing insight on new mechanisms of action.
Keywords: Drosophila; Shaker channel; NMDA receptor; bipolar disorder; glutamate; memantine.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
There are no conflicts among the authors.
Figures




References
-
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413‐426. - PubMed
-
- de Bartolomeis A, Buonaguro EF, Iasevoli F, Tomasetti C. The emerging role of dopamine–glutamate interaction and of the postsynaptic density in bipolar disorder pathophysiology: implications for treatment. J Psychopharmacol. 2014;28:505‐526. - PubMed
-
- APA Work Group on Psychiatric Evaluation . The American Psychiatric Association practice guidelines for the psychiatric evaluation of adults / APA. 3rd ed. Work Group on Psychiatric Evaluation, Joel J. Silverman, chair [and eleven others]. American Psychiatric Association; 2016.
-
- Sepede G, Chiacchiaretta P, Gambia F, et al. Bipolar disorder with and without a history of psychotic features: fMRI correlates of sustained attention. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

