Heterogeneous Treatment Effects of Therapeutic-Dose Heparin in Patients Hospitalized for COVID-19
- PMID: 36942550
- PMCID: PMC10031504
- DOI: 10.1001/jama.2023.3651
Heterogeneous Treatment Effects of Therapeutic-Dose Heparin in Patients Hospitalized for COVID-19
Abstract
Importance: Randomized clinical trials (RCTs) of therapeutic-dose heparin in patients hospitalized with COVID-19 produced conflicting results, possibly due to heterogeneity of treatment effect (HTE) across individuals. Better understanding of HTE could facilitate individualized clinical decision-making.
Objective: To evaluate HTE of therapeutic-dose heparin for patients hospitalized for COVID-19 and to compare approaches to assessing HTE.
Design, setting, and participants: Exploratory analysis of a multiplatform adaptive RCT of therapeutic-dose heparin vs usual care pharmacologic thromboprophylaxis in 3320 patients hospitalized for COVID-19 enrolled in North America, South America, Europe, Asia, and Australia between April 2020 and January 2021. Heterogeneity of treatment effect was assessed 3 ways: using (1) conventional subgroup analyses of baseline characteristics, (2) a multivariable outcome prediction model (risk-based approach), and (3) a multivariable causal forest model (effect-based approach). Analyses primarily used bayesian statistics, consistent with the original trial.
Exposures: Participants were randomized to therapeutic-dose heparin or usual care pharmacologic thromboprophylaxis.
Main outcomes and measures: Organ support-free days, assigning a value of -1 to those who died in the hospital and the number of days free of cardiovascular or respiratory organ support up to day 21 for those who survived to hospital discharge; and hospital survival.
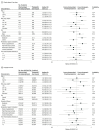
Results: Baseline demographic characteristics were similar between patients randomized to therapeutic-dose heparin or usual care (median age, 60 years; 38% female; 32% known non-White race; 45% Hispanic). In the overall multiplatform RCT population, therapeutic-dose heparin was not associated with an increase in organ support-free days (median value for the posterior distribution of the OR, 1.05; 95% credible interval, 0.91-1.22). In conventional subgroup analyses, the effect of therapeutic-dose heparin on organ support-free days differed between patients requiring organ support at baseline or not (median OR, 0.85 vs 1.30; posterior probability of difference in OR, 99.8%), between females and males (median OR, 0.87 vs 1.16; posterior probability of difference in OR, 96.4%), and between patients with lower body mass index (BMI <30) vs higher BMI groups (BMI ≥30; posterior probability of difference in ORs >90% for all comparisons). In risk-based analysis, patients at lowest risk of poor outcome had the highest propensity for benefit from heparin (lowest risk decile: posterior probability of OR >1, 92%) while those at highest risk were most likely to be harmed (highest risk decile: posterior probability of OR <1, 87%). In effect-based analysis, a subset of patients identified at high risk of harm (P = .05 for difference in treatment effect) tended to have high BMI and were more likely to require organ support at baseline.
Conclusions and relevance: Among patients hospitalized for COVID-19, the effect of therapeutic-dose heparin was heterogeneous. In all 3 approaches to assessing HTE, heparin was more likely to be beneficial in those who were less severely ill at presentation or had lower BMI and more likely to be harmful in sicker patients and those with higher BMI. The findings illustrate the importance of considering HTE in the design and analysis of RCTs.
Trial registration: ClinicalTrials.gov Identifiers: NCT02735707, NCT04505774, NCT04359277, NCT04372589.
Conflict of interest statement
Figures



Comment in
-
Toward Personalizing Care: Assessing Heterogeneity of Treatment Effects in Randomized Trials.JAMA. 2023 Apr 4;329(13):1063-1065. doi: 10.1001/jama.2023.3576. JAMA. 2023. PMID: 36942555 No abstract available.
-
Treatment Effects of Therapeutic-Dose Heparin in Patients Hospitalized for COVID-19.JAMA. 2023 Aug 1;330(5):471-472. doi: 10.1001/jama.2023.9535. JAMA. 2023. PMID: 37526726 No abstract available.
References
-
- Sholzberg M, Tang GH, Rahhal H, et al. ; RAPID Trial Investigators . Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375(2400):n2400. doi:10.1136/bmj.n2400 - DOI - PMC - PubMed
-
- Sadeghipour P, Talasaz AH, Rashidi F, et al. ; INSPIRATION Investigators . Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620-1630. doi:10.1001/jama.2021.4152 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

