Sepsis-induced endothelial dysfunction drives acute-on-chronic liver failure through Angiopoietin-2-HGF-C/EBPβ pathway
- PMID: 36943063
- PMCID: PMC10440279
- DOI: 10.1097/HEP.0000000000000354
Sepsis-induced endothelial dysfunction drives acute-on-chronic liver failure through Angiopoietin-2-HGF-C/EBPβ pathway
Abstract
Background and aims: Acute-on-chronic liver failure (ACLF) is an acute liver and multisystem failure in patients with previously stable cirrhosis. A common cause of ACLF is sepsis secondary to bacterial infection. Sepsis-associated ACLF involves a loss of differentiated liver function in the absence of direct liver injury, and its mechanism is unknown. We aimed to study the mechanism of sepsis-associated ACLF using a novel mouse model.
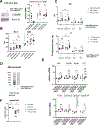
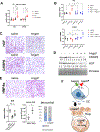
Approach and results: Sepsis-associated ACLF was induced by cecal ligation and puncture procedure (CLP) in mice treated with thioacetamide (TAA). The combination of TAA and CLP resulted in a significant decrease in liver synthetic function and high mortality. These changes were associated with reduced metabolic gene expression and increased CCAAT enhancer binding protein beta (C/EBPβ) transcriptional activity. We found that C/EBPβ binding to its target gene promoters was increased. In humans, C/EBPβ chromatin binding was similarly increased in the ACLF group compared with control cirrhosis. Hepatocyte-specific Cebpb knockout mice had reduced mortality and increased gene expression of hepatocyte differentiation markers in TAA/CLP mice, suggesting that C/EBPβ promotes liver failure in these mice. C/EBPβ activation was associated with endothelial dysfunction, characterized by reduced Angiopoietin-1/Angiopoietin-2 ratio and increased endothelial production of HGF. Angiopoietin-1 supplementation or Hgf knockdown reduced hepatocyte C/EBPβ accumulation, restored liver function, and reduced mortality, suggesting that endothelial dysfunction induced by sepsis drives ACLF through HGF-C/EBPβ pathway.
Conclusions: The transcription factor C/EBPβ is activated in both mouse and human ACLF and is a potential therapeutic target to prevent liver failure in patients with sepsis and cirrhosis.
Copyright © 2023 American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures






References
-
- Moreau R, Gao B, Papp M, Bañares R, Kamath PS. Acute-on-chronic liver failure: A distinct clinical syndrome. J Hepatol 2021;75 Suppl 1:S27–s35. - PubMed
-
- Heron M Deaths: Leading Causes for 2019. Natl Vital Stat Rep 2021;70:1–114. - PubMed
-
- Solé C, Solà E. Update on acute-on-chronic liver failure. Gastroenterol Hepatol 2018;41:43–53. - PubMed
-
- Mucke MM, Rumyantseva T, Mucke VT, Schwarzkopf K, Joshi S, Kempf VAJ, Welsch C, et al. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int 2018;38:645–653. - PubMed
-
- Li X, Wang LK, Wang LW, Han XQ, Yang F, Gong ZJ. Blockade of high-mobility group box-1 ameliorates acute on chronic liver failure in rats. Inflamm Res 2013;62:703–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

